©The Author(s) 2020.
World J Gastrointest Endosc. Aug 16, 2020; 12(8): 241-255
Published online Aug 16, 2020. doi: 10.4253/wjge.v12.i8.241
Published online Aug 16, 2020. doi: 10.4253/wjge.v12.i8.241
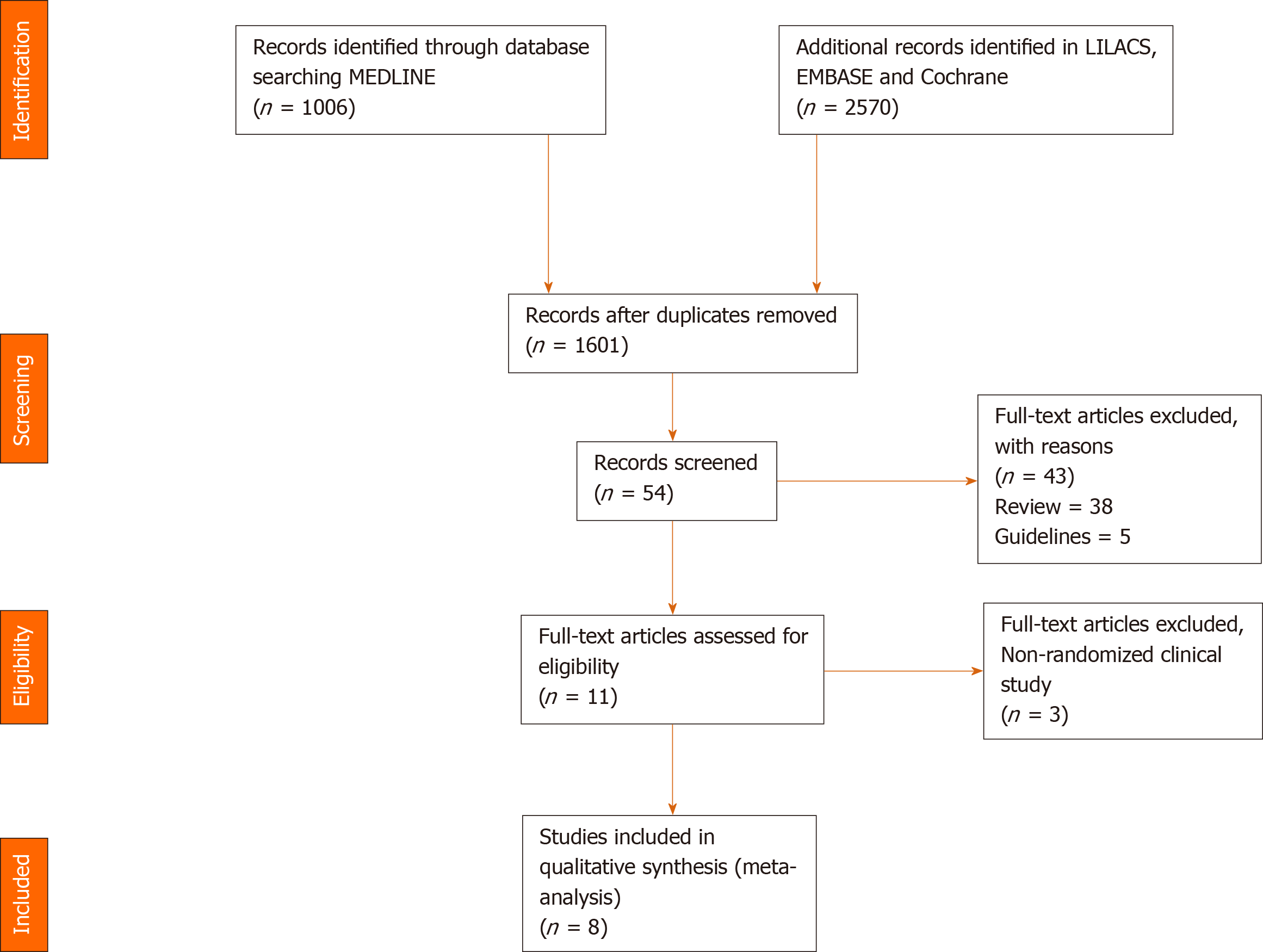
Figure 1 Flow chart of study selection.
Cochrane CENTRAL: Cochrane Central Register of Controlled Trials; Propofol vs midazolam sedation for elective endoscopy in patients with cirrhosis.
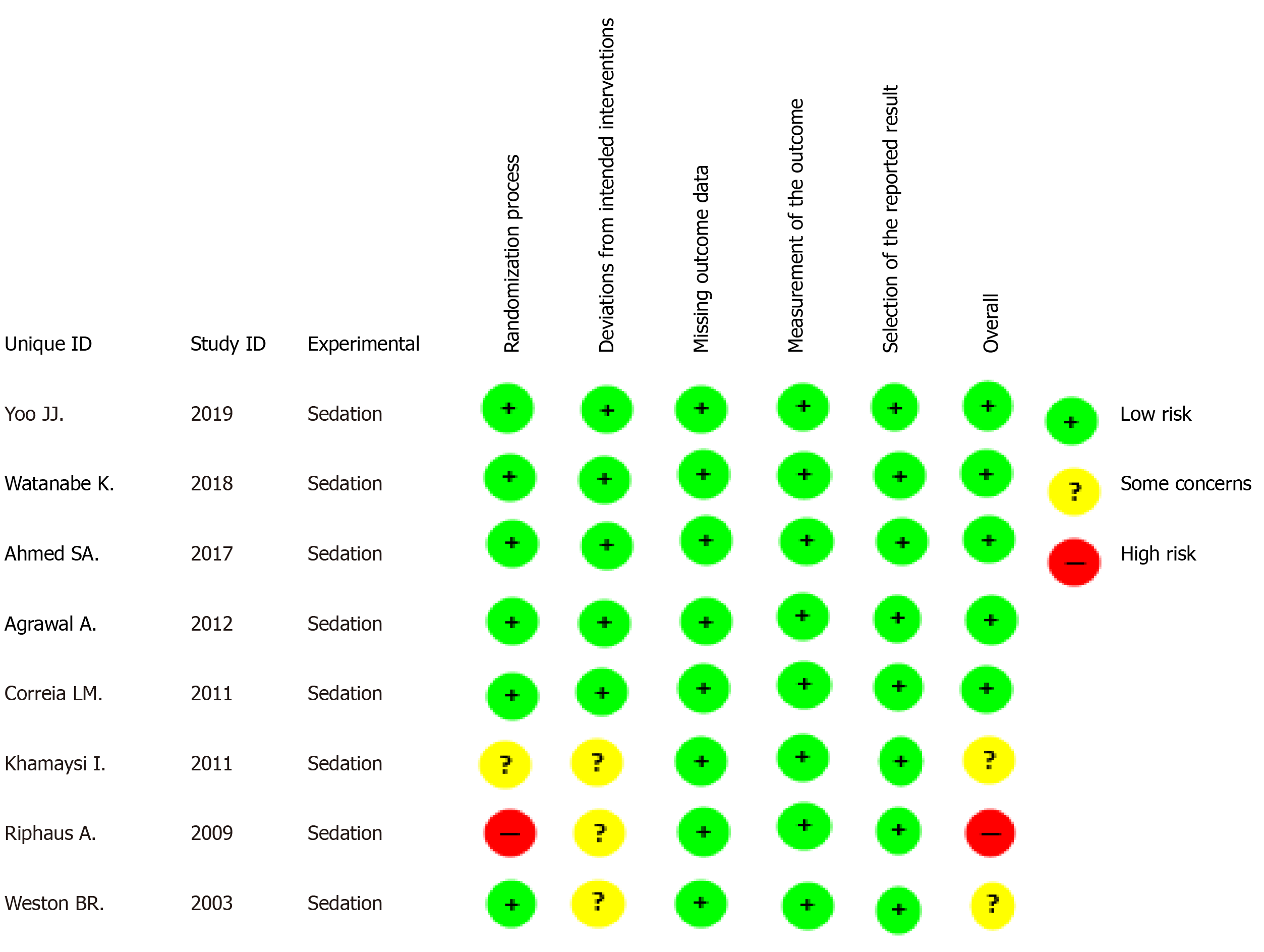
Figure 2 Overall risk of bias.
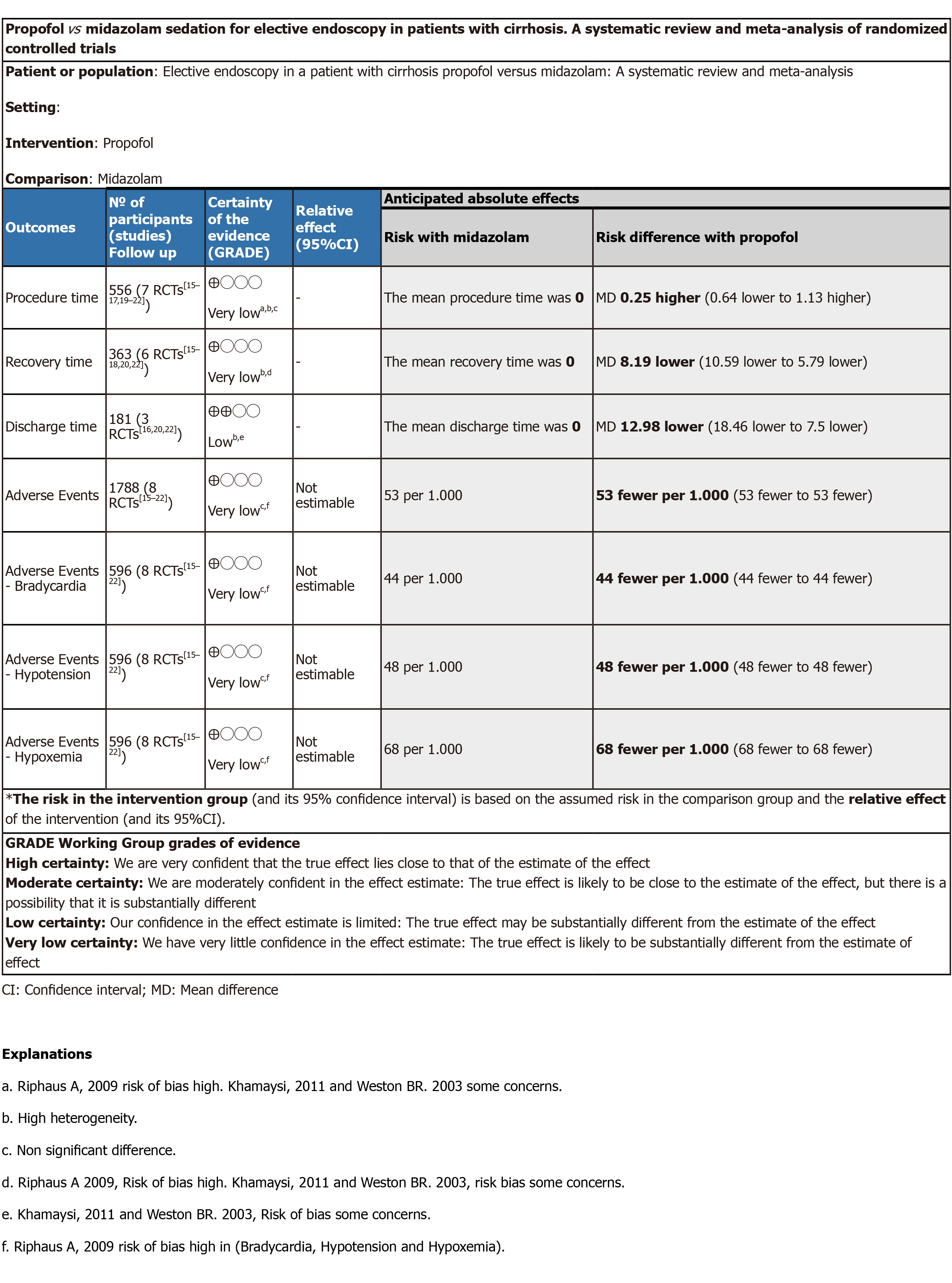
Figure 3 GRADEpro.
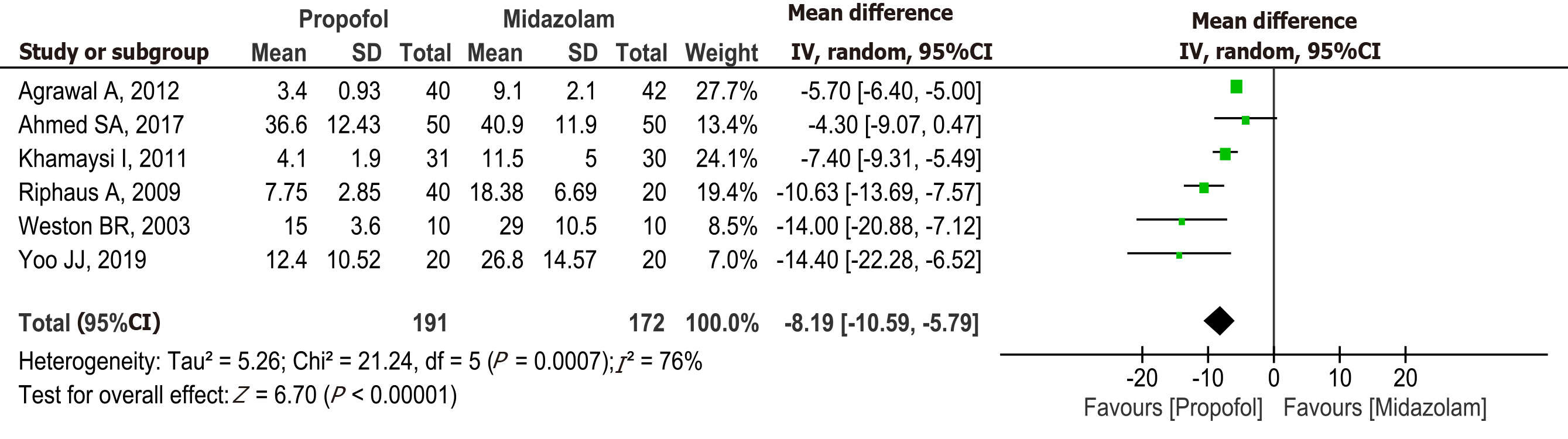
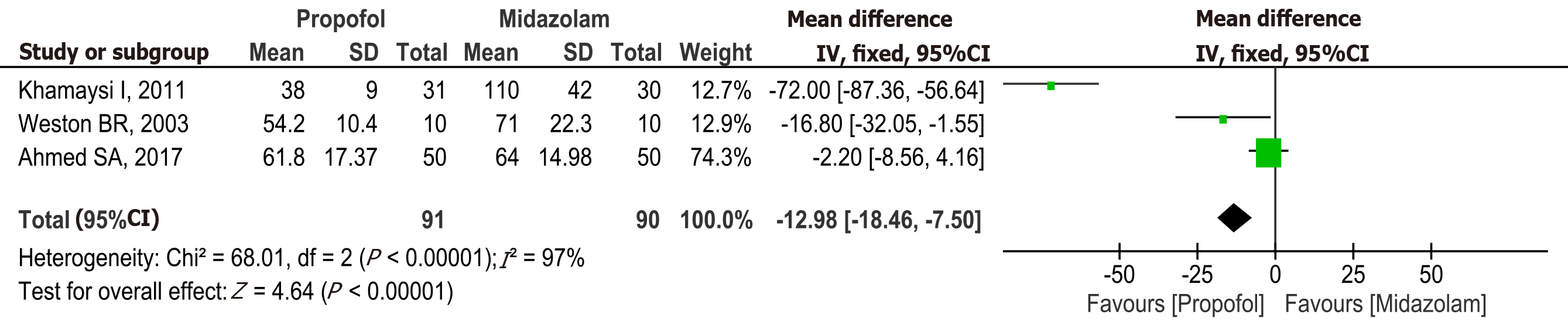
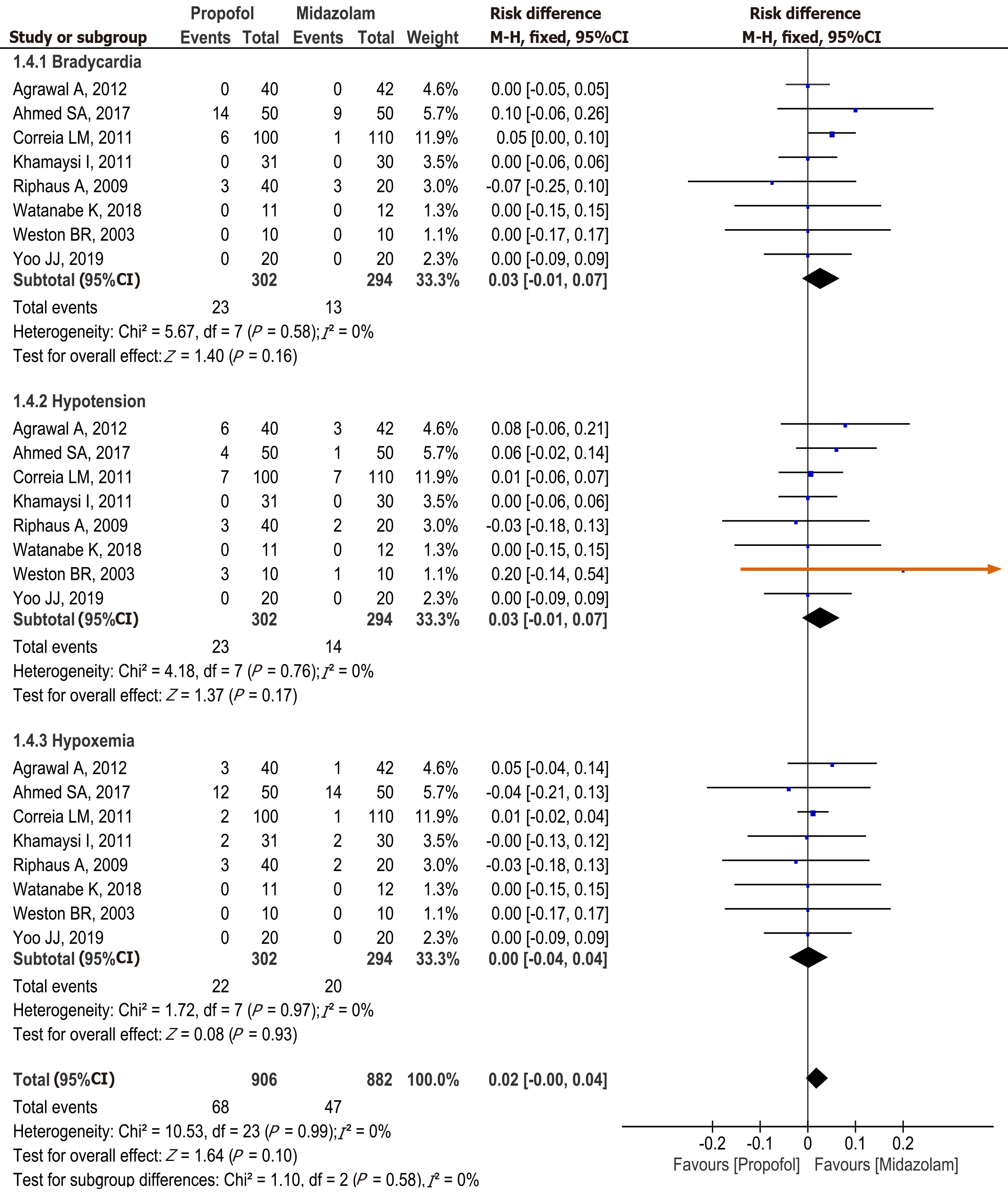
Propofol vs midazolam sedation for elective endoscopy in patients with cirrhosis. A systematic review and meta-analysis of randomized controlled trials. aRiphaus A, 2009 risk of bias high. Khamaysi, 2011 and Weston BR. 2003 some concerns. bHigh heterogeneity. cNon significant difference. dRiphaus A 2009, Risk of bias high. Khamaysi, 2011 and Weston BR. 2003, risk bias some concerns. eKhamaysi, 2011 and Weston BR. 2003, Risk of bias some concerns. fRiphaus A, 2009 risk of bias high in (Bradycardia, Hypotension and Hypoxemia). CI: Confidence interval; MD: Mean difference.
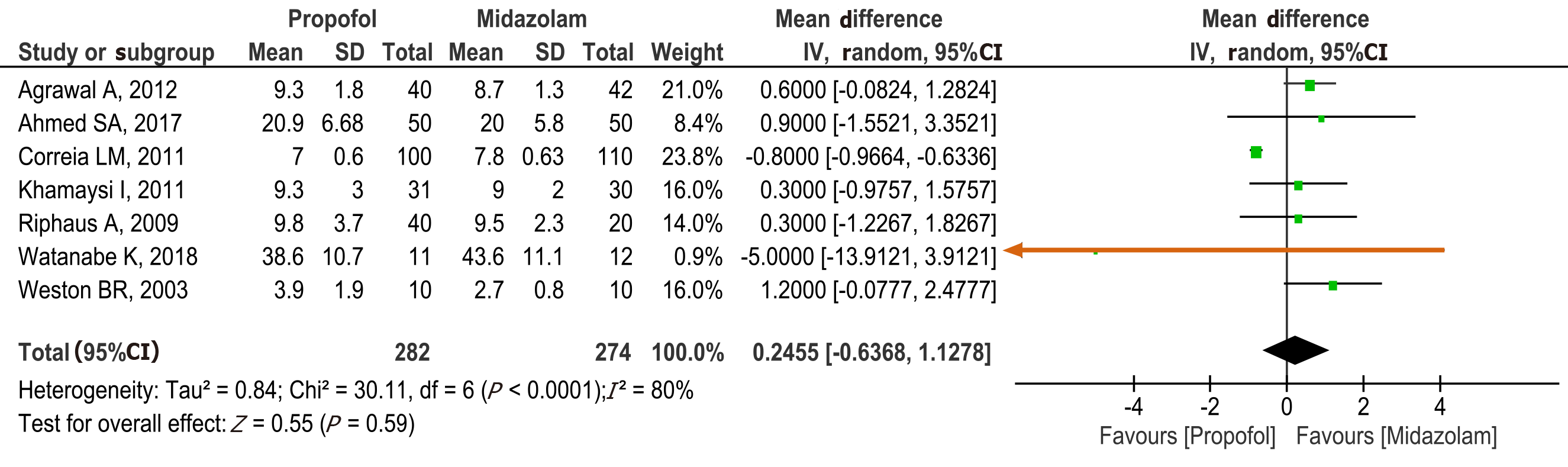
Figure 4 Forest plot comparing procedure time between propofol and midazolam group for sedation during elective upper gastrointestinal endoscopy in patients with cirrhosis.
Figure 5 Forest plot comparing recovery time between propofol and midazolam group for sedation during elective upper gastrointestinal endoscopy in patients with cirrhosis.
Figure 6 Forest plot comparing discharge time between propofol and midazolam group for sedation during elective upper gastrointestinal endoscopy in patients with cirrhosis.
Figure 7 Forest plot comparing adverse events between propofol and midazolam group for sedation during elective upper gastrointestinal endoscopy in patients with cirrhosis.
- Citation: Guacho JAL, de Moura DTH, Ribeiro IB, da Ponte Neto AM, Singh S, Tucci MGB, Bernardo WM, de Moura EGH. Propofol vs midazolam sedation for elective endoscopy in patients with cirrhosis: A systematic review and meta-analysis of randomized controlled trials. World J Gastrointest Endosc 2020; 12(8): 241-255
- URL: https://www.wjgnet.com/1948-5190/full/v12/i8/241.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i8.241