Published online Feb 27, 2019. doi: 10.4254/wjh.v11.i2.138
Peer-review started: October 6, 2018
First decision: October 26, 2018
Revised: November 1, 2018
Accepted: December 4, 2018
Article in press: December 5, 2018
Published online: February 27, 2019
Processing time: 145 Days and 3.6 Hours
Non-alcoholic fatty liver disease (NAFLD) is increasingly recognized as a significant liver disease, and it covers the disease spectrum from simple steatosis with a risk of development of non-alcoholic steatohepatitis (NASH) to fibrosis, subsequent cirrhosis, end-stage liver failure, and liver cancer with a potential need for liver transplantation. NAFLD and NASH are closely related to obesity, metabolic syndrome, and type 2 diabetes (T2D). The role of gut hormones, especially glucagon-like peptide 1 (GLP-1), is important in NAFLD. Bariatric surgery has the potential for inducing great weight loss and may improve the symptoms of metabolic syndrome and T2D. Recent data demonstrated significant effects of bariatric surgery on GLP-1 and other gut hormones and important lipid metabolic and inflammatory abnormalities in the pathophysiology of NAFLD. Therefore, bariatric surgery may reverse the pathological liver changes in NAFLD and NASH patients. In the present review, we describe NAFLD and NASH pathophysiology and the primary effects of bariatric surgery on metabolic pathways. We performed a systematic review of the beneficial and harmful effects and focused on changes in liver disease severity in NAFLD and NASH patients. The specific focus was liver histopathology as assessed by the invasive liver biopsy. Additionally, we reviewed several non-invasive methods used for the assessment of liver disease severity following bariatric surgery.
Core tip: Non-alcoholic fatty liver disease (NAFLD) is a significant liver disease with risks of steatohepatitis (NASH), fibrosis, and cirrhosis. NAFLD and NASH are closely related to obesity, the metabolic syndrome, and type 2 diabetes (T2D). Bariatric surgery induces weight loss and improves the features of the metabolic syndrome and T2D. Surgery may reverse pathological liver changes. In the present review, we focus on the primary effects of bariatric surgery on metabolic pathways and systematically reviews the effects of bariatric surgery on changes in liver disease severity in NAFLD and NASH patients.
- Citation: Laursen TL, Hagemann CA, Wei C, Kazankov K, Thomsen KL, Knop FK, Grønbæk H. Bariatric surgery in patients with non-alcoholic fatty liver disease - from pathophysiology to clinical effects. World J Hepatol 2019; 11(2): 138-149
- URL: https://www.wjgnet.com/1948-5182/full/v11/i2/138.htm
- DOI: https://dx.doi.org/10.4254/wjh.v11.i2.138
Alarming increases in obesity and diabetes coupled with changes towards unhealthy lifestyles and dietary habits have contributed to a dramatic increase in non-alcoholic fatty liver disease (NAFLD), which affects 25%-30% of the general population[1]. NAFLD is most often asymptomatic and consists of a disease spectrum ranging from simple steatosis (NAFL) and steatohepatitis (NASH) to fibrosis and cirrhosis, with significant clinical consequences, including but not limited to ascites, varices, hepatic encephalopathy, liver cancer and liver transplantation or early death[2]. NASH may develop with hepatic inflammation, hepatocellular injury, macrophage and hepatic stellate cell activation in patients with simple steatosis. If untreated, NASH may progress to cirrhosis. NASH-induced cirrhosis is fast becoming the most common indication for liver transplantation, which is strongly associated with poor quality of life[3].
NAFLD is closely related to obesity and type 2 diabetes mellitus (T2D), and it is often termed the hepatic manifestation of metabolic syndrome[4,5]. The prevalence of NASH increases with components of the metabolic syndrome in T2D[6]. Available epidemiological data suggest a prevalence of NAFLD of 40%-70% in European T2D patients[1]. Insulin resistance is more prevalent in NASH patients than patients with simple steatosis[7], and patients with NAFLD without T2D exhibit decreased insulin sensitivity[8].
Bariatric surgery is an efficient treatment of obesity and causes sustained weight loss with potential reductions in hepatic fat, inflammation and fibrosis[9,10]. Roux-en-Y gastric bypass surgery (RYGB) is the most effective treatment for obesity[11]. This procedure improves glycaemic control, and T2D patients experience a reduced need for antidiabetic medication within days after surgery[9]. Sleeve gastrectomy (SG) and adjustable gastric banding (AGB) are alternative surgical approaches that significantly reduce gastric volume without changing the upper gastrointestinal tract anatomy. SG has gained popularity in recent years and been established as a comparable method to RYGB. In contrast, AGB is associated with less weight loss than RYGB surgery. The indication for bariatric surgery is severe obesity with or without T2D and/or other comorbidities[9].
We searched the following databases: MEDLINE Ovid (1946 to June 2018), Science Citation Index Expanded (Web of Science; 1900 to June 2018), and PubMed [Bethesda (MD): National Library of Medicine (US) 1966 to June 2018]. The following search terms were used: “Non-alcoholic fatty liver disease” (MeSH, all fields) or “Non-alcoholic steatohepatitis” (all fields) and “bariatric surgery (MeSH, all fields). Only English language articles were selected, and case reports were excluded. Full-text evaluation was performed, and references from relevant manuscripts were reviewed manually for additional manuscripts. This search strategy identified 404 studies at the end of June 2018. Studies were included in our comprehensive review if they were published between January 2010 and June 2018, were prospective or retrospective observational studies and if they evaluated the effects of bariatric surgery on histopathological NAFLD. In total, we ended up with 13 studies.
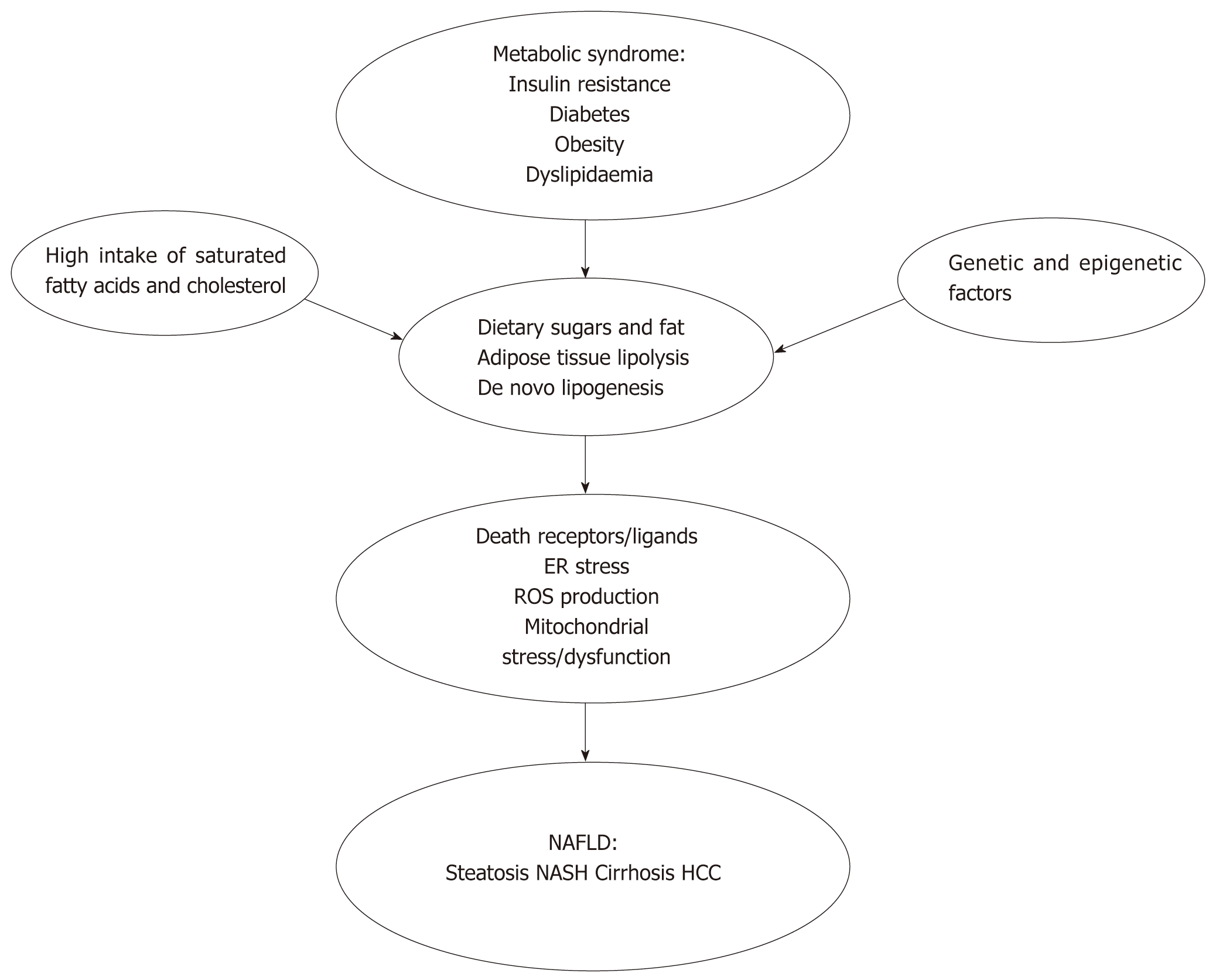
The pathogenic mechanisms for the development and progression of NAFLD are complex and multifactorial[12] (Figure 1). Genetic and epigenetic factors affect the development of NAFLD and NASH progression and potentially influence or modify risk factors[13-16]. Dietary sugars, fat, adipose tissue lipolysis, and de novo lipogenesis contribute to increased hepatic fat influx and accumulation in obese patients[17]. Obese patients exhibit increased adipose tissue mass, which leads to adipocyte dysfunction, including insulin resistance, increased lipolysis and apoptosis, and results in local inflammation and cytokine release. Insulin resistance reduces insulin-induced inhibition of lipolysis, and negatively affects the ability of the adipose tissue to store fat, which results in increased free fatty acids in the blood. Insulin resistance induces further insulin secretion, which instigates high blood insulin levels[18,19].
Hepatic de novo lipogenesis is also augmented in obese patients, partially due to enzyme upregulation induced by hyperinsulinaemia, elevated plasma glucose levels and endoplasmic reticulum (ER) stress[20-22]. Lipid accumulation in the liver primarily consists of triglycerides, which may not be hepatotoxic per se, but reflects the general inability of hepatocytes to handle fatty acids and leads to the concurrent accumulation of toxic lipid metabolites[23-25]. Long-chain saturated fatty acids resulting from de novo lipogenesis specifically harm liver cells via triggering the formation of reactive oxygen species, which highly contribute to hepatic lipotoxicity[19].
Activation of death receptors and their ligands, induction of ER stress, the production of reactive oxygen species and mitochondrial stress and dysfunction lead to hepatocyte injury and death with subsequent release of proteins, debris, etc., which are collectively defined as damage-associated molecular patterns (DAMPs)[26,27]. Fatty acids, DAMPs and pathogen-associated molecular patterns (PAMPs), e.g., bacteria and endotoxins, likely originating from a leaky gut, are the primary inducers of hepatic inflammation, which involves activation of resident and recruited macrophages in the liver[28,29]. Macrophage activation results in pro-inflammatory cytokine secretion and the activation of hepatic stellate cells into myofibroblasts, which secrete the collagen that contributes to extracellular matrix formation. Myofibroblasts are also directly responsive to cytokines, DAMPs and PAMPs, thus further propagating fibrosis formation[30].
Bariatric surgery has tremendous effects on metabolic functions. Buchwald et al[31] performed a meta-analysis of 136 studies that assessed the impact of bariatric surgery on metabolic outcomes and reported a complete resolution of T2D in more than 75% of diabetic patients and an excessive weight loss of almost 60%. A review of key results from the Swedish Obese Subjects study reported a 72% remission rate of T2D two years post-bariatric surgery[32]. Several other studies demonstrated that RYGB and SG were superior to conventional pharmacological therapy in achieving glycaemic control in T2D patients[33-35].
NAFLD is closely associated with obesity and T2D, and the mechanisms implicated in improving obesity and T2D following bariatric surgery likely play important roles in the resolution of NAFLD. Several mechanisms independent of weight loss are involved in the initial metabolic responses to RYGB and SG procedures and maintaining these improvements over the long term, despite the obvious causality between weight loss and improvements in T2D and NAFLD.
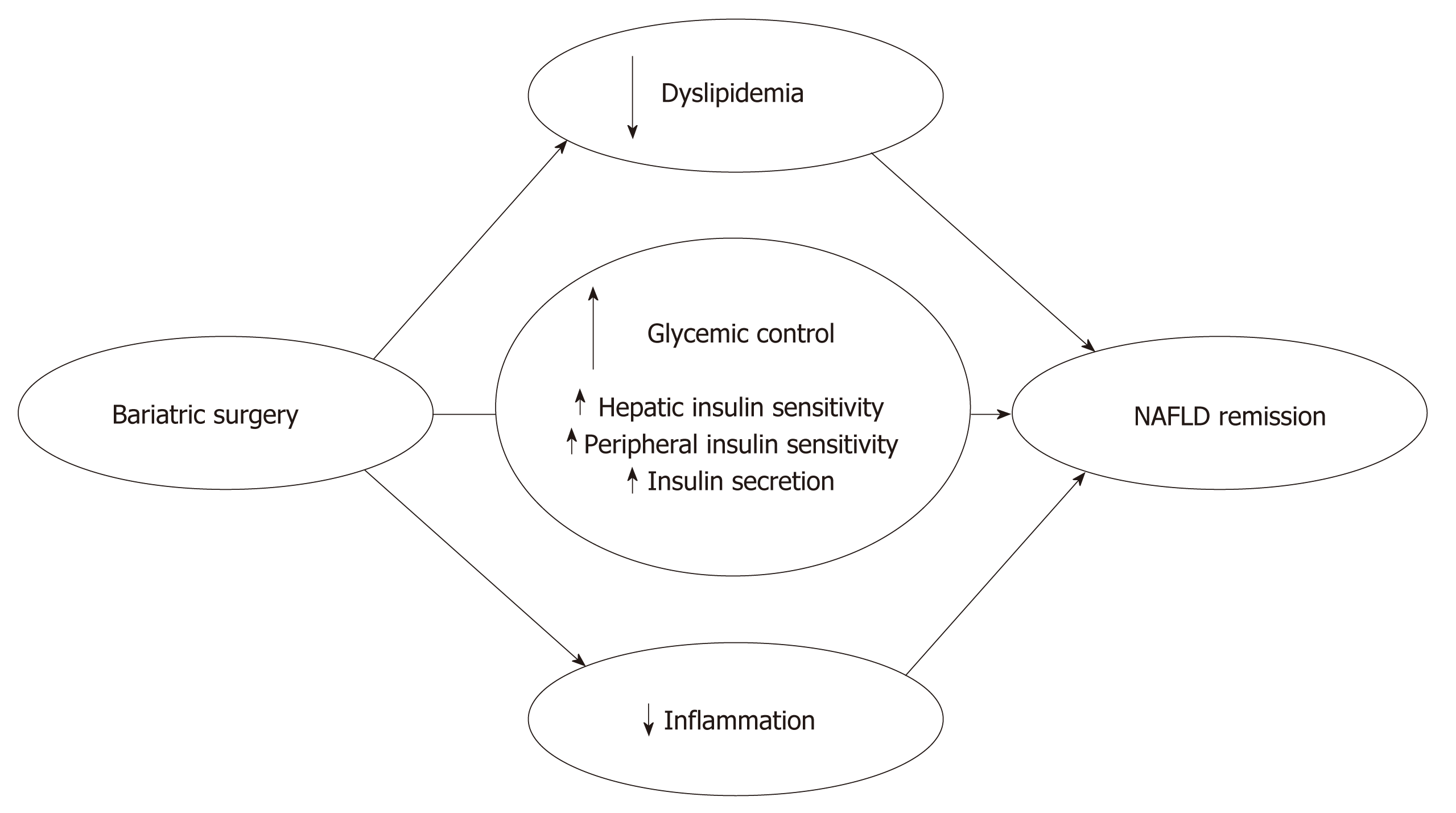
The almost immediate metabolic benefits of bariatric surgery, independent of any significant weight loss, have been known for decades[36] but are striking nonetheless[31,37,38]. Three primary mechanisms are involved in the improved glycaemic control associated with the RYGB and SG procedures: (1) early improved hepatic insulin sensitivity due to the post-surgery calorie restriction; (2) late improved peripheral insulin sensitivity due to weight loss; and (3) improved post-prandial insulin secretion due to a rise in glucagon-like peptide 1 (GLP-1) secretion (Figure 2). Several studies investigated the post-surgical metabolic changes, and whether the change in the release of gut hormones or surgery-induced restriction of food intake provides the essential effects on glycaemic control remains controversial. Jørgensen et al[38,39] found an increase in post-prandial GLP-1, insulin secretion and hepatic insulin sensitivity within days after RYGB, and this increase was sustained for at least 1 year in diabetic and non-diabetic-matched subjects, which is consistent with other RYGB and SG studies[37,40,41]. The GLP-1 increase represents a powerful beta-cell stimulus and is explained by the accelerated entry of nutrients into the small intestine after RYGB[42,43] and SG[44], which increases the glucose absorption rate in the L cells responsible for the GLP-1 secretion[45]. The accelerated transport of nutrients into more distal parts of the small intestine may further explain the exaggerated GLP-1 response because a higher density of L cells are found in this area[46]. Notably, GLP-1 may have beneficial gene-regulatory effects on fatty acid oxidation and insulin sensitivity in hepatocytes[47], but these findings require confirmation in humans. Postprandial glucagon responses also increase post-operatively[38,48] despite the inhibitory effects of GLP-1 on glucagon secretion[39]. This paradoxical effect (in the context of improved glucose metabolism) may represent gut-derived glucagon[42] and may exert an attenuating effect on glycaemic control post-surgery.
Steven et al[49], among others[50], demonstrated that the reduced liver fat content from calorie restriction explained the early improvement in hepatic insulin sensitivity, as illustrated using magnetic resonance imaging. These data suggest that significant caloric restriction explains the almost immediate metabolic benefits from bariatric surgery due to improved liver function[49,51]. Vetter et al[48] demonstrated that the improvements in liver insulin sensitivity from RYGB exceeded lifestyle modifications. However, blockade of the GLP-1 receptor using the antagonist exendin9–39 consistently lowered insulin secretion after RYGB and SG[39,52], and it reversed the postprandial hyperinsulinaemic hypoglycaemia observed post-operatively[38], which confirmed the causative role of GLP-1 in beta-cell stimulation[53-55]. Calorie restriction is the leading mechanism of the early metabolic changes after bariatric surgery, but the gut hormones, particular GLP-1, remain crucial for the fine-tuning of the glycaemic control and post-prandial insulin secretion[56].
A hyperinsulinaemic clamp study of Bojsen-Møller et al[37] demonstrated that peripheral insulin sensitivity, as assessed using glucose disposal and suppression of fatty acids, increased after three months in relation to the surgery-induced weight loss. Lifestyle modifications exert the same powerful effects on glycaemic control and NAFLD[48,57], but this intervention generally fails to sustain the short-term weight loss[32]. Notably, bariatric surgery is superior in maintaining calorie restriction and long-term weight loss, which is facilitated at least partially by the reduced appetite observed post-operatively[58,59]. The long-term reduced appetite may be attributable to a favourable shift in the anorectic gut hormones GLP-1 and peptide-YY (PYY)[58,60]. An antagonist study using exendin9–39 and a dipeptidyl peptidase 4 (DPP-4) inhibitor (blocking the DPP-4-mediated formation of active PYY from its precursor) in RYGB-operated subjects demonstrated a 20% increase in food intake[61]. Several other factors are candidates for the long-term metabolic improvements of bariatric surgery, including ghrelin[62], adiponectin[63], increased plasma bile acids[64] and changed intestinal microbiota[65], but proof of causality for these factors remains to be established in humans.
Bariatric surgery affects NAFLD not only through a rapid and substantial weight loss but also via simultaneous effects on important lipid metabolic and inflammatory pathways involved in NAFLD pathophysiology[66,67]. Bariatric surgery promotes changes in three crucial metabolic areas influencing NAFLD: improved glucose homeostasis, improved lipid metabolism and reduced inflammatory activity (Figure 2). These effects are followed by significant effects on liver abnormalities in NAFLD and NASH patients.
A systematic review and meta-analysis[68] in 2008 assessed the histological effects of bariatric surgery in NAFLD patients. This review concluded that the features of steatosis, steatohepatitis, and fibrosis improve or resolve in most patients following weight loss after bariatric surgery. A Cochrane review in 2010 reported more discrete conclusions[69]. These authors were not able to identify any randomized clinical trials at that time and advised caution even though several reports demonstrated potential favourable effects of bariatric surgery. Most studies reported beneficial effects on steatosis, and more than half of the studies demonstrated significant improvements in histological inflammation. Six studies demonstrated improvement in fibrosis scores, but 4 studies[70-73] reported some worsening of fibrosis.
Our literature search did not identify any randomized controlled trials that assessed the hepatic histological effects of bariatric surgery in NAFLD patients. We performed a comprehensive review of prospective and retrospective observational studies published since 2010 to evaluate the effects of bariatric surgery on histopathological NAFLD (Table 1). We identified 13 studies: Eight studies with prospective designs, two studies with retrospective designs and three studies in which the design was not obvious. The types of surgery included RYGB, AGB and SG, and most studies assessed the effect of RYGB. The sample size ranged from 9 to 578 patients. The studies clustered into three categories based on participant numbers: Two large studies with more than 150 participants[74,75], three studies with 50-150 participants[10,76,77] and eight small studies with less than 50 participants[78-85].
| Ref. | Design | Patients with follow-up | Surgical inter-vention | Steatosis | Inflammation | Fibrosis | Any cases of worsening | Mean/median follow-up in months |
| Weiner et al[76], 2010 | Missing | 116 | RYGB, AGB, BPD-DS | Improved, 70% complete resolution | Improved, 86% complete resolution | Improved | No | 19.4 (± 8.3) |
| Moretto et al[77], 2012 | Retrospective | 78 | Gastric bypass | Improved | Improved ballooning, 55% complete resolution of NASH | Trend for improvement | Yes | Unknown |
| Vargas et al[78], 2012 | Prospective | 26 | RYGB | Improved | Improved, 84% complete resolution of NASH | Improved | No | 16 (± 3) |
| Tai et al[79], 2012 | Prospective | 21 | RYGB | Improved, 95% complete resolution | Improved, 100% complete resolution of NASH | Improved | Yes | 12 |
| Caiazzo et al[74], 2014 | Prospective | 578 (1 yr), 413 (5 yr) | RYGB, AGB | Improved | Improved | Improved | NA | 12 and 60 |
| Lassailly et al[10], 2015 | Prospective | 82 | Gastric bypass, AGB, SG, PBD-DS | Improved | Improved, 85% complete resolution of NASH | Improved | NA | 12 |
| Praveen et al[80], 2015 | Prospective | 30 | RYGB, SG | Improved in 97% | Improved in 46% | Improved in 46% | No | 6 |
| Taitano et al[75], 2015 | Missing | 160 | RYGB, AGB | Improved, complete resolution in 73% | Improved, 88% complete resolution of NASH | Improved, 53% complete resolution | Yes | 31 (± 26) |
| Schneck et al[81], 2016 | Missing | 9 | RYGB | Improved in all patients | Improved in all patients | Improved | Yes | 55 (44-75) |
| Froylich et al[82], 2016 | Retrospective | 25 | RYGB, SG | Improved | Improved | Trend for improvement | NA | 18 |
| Aldoheyan et al[83], 2017 | Prospective | 27 | SG | Improved | Improved | Improved | NA | 3 |
| Manco et al[84], 2017 | Prospective | 20 | SG (n = 20) vs IGWLD, NSWL | Improved | Improved, 100% complete resolution of NASH | Improved | NA | 12 |
| Garg et al[85], 2018 | Prospective | 32 | RYGB, AGB, SG | Improved | Improved | Improved | NA | 12 |
In the largest study by Caiazzo et al[74] including more than 500 patients, the effects of RYGB and AGB were compared. Improvement and resolution of steatosis, inflammation and fibrosis were observed one and five years after both types of surgery. Biopsies at all three time points (before and 1 and 5 years post-surgery) were available in 315 patients, and the authors did not describe any cases of worsening. The best effects on weight loss and liver histology were achieved in the RYGB patients, and the primary effect derived from a greater weight loss but additionally explained by a more positive influence on glucose and lipid metabolism.
The second largest study of 160 patients undergoing RYGB or ABG with a mean follow-up of 31 mo demonstrated resolution or improvement of steatosis and inflammation in most patients[75]. Fibrosis resolved or improved in more than half of the patients. However, 8% of the patients progressed or developed steatosis de novo after surgery. Portal inflammation worsened in 10% and developed de novo in 27% of the patients, and 16% developed lobular inflammation after surgery. Fibrosis progressed in 12% of the patients with pre-surgery fibrosis and another 21% developed de novo fibrosis. Three patients developed NASH de novo after surgery.
In general, all of the smaller studies reported improvements in steatosis, inflammation and fibrosis. However, three of these studies found a worsening of some histological features, e.g., inflammation[79] and fibrosis[77,81], and three other studies reported no worsening[76,78,80]. The remaining studies did not describe worsening in any patients. The histological liver changes were accompanied with beneficial effects on metabolic syndrome[78,79], hypertension, dyslipidaemia and obstructive sleep apnoea[84]. Most studies performed follow-up biopsies after one year or later after surgery, but two studies performed follow-up at three[83] and six[80] months. Notably, the effects of surgery were visible at these time points, even for fibrosis.
Lassailly et al[10] investigated differences in patients with resolution of NASH one year after surgery and patients with persistent NASH and found that these patients had lost significantly less weight and were more frequently classified with a refractory IR profile, which suggests that the weight loss was of primary importance.
Other studies used diverse non-invasive methods to examine the hepatic effects of bariatric surgery and found improvements in general. Several studies investigated how bariatric surgery affected the levels of circulating liver transaminases, in general reporting favourable effects, as summarized in a meta-analysis[86]. However, transaminases exhibit limited accuracy for the prediction of NASH severity.
Several studies used non-invasive fibrosis scores. One study demonstrated decreases in NAFLD fibrosis scores, ratio of aminotransferase (AST) to alanine aminotransferase (ALT), AST-to-platelet ratio index (APRI), and BARD score (BMI, ASAT/ALAT ratio, and the presence of T2D) one year after surgery[87]. Decreases in the NAFLD fibrosis score were confirmed in several other studies. Two studies of RYGB found a significant decrease 12 mo[88] and 36 mo after surgery[89], but higher levels in patients who regained weight after the initial weight loss. One study of 56 adolescents described a decrease in NAFLD fibrosis score one and two years after AGB[90]. However, Simo et al[91] questioned the feasibility of the NAFLD fibrosis score in relation to RYGB because several patients were wrongly classified or ended in a group of indeterminate classification. None of these studies included pre- or post-surgery biopsies as golden standards for treatment effects.
Forty-two patients who underwent diverse types of surgery were followed up with liver stiffness measurements and controlled attenuation parameter (CAP) using transient elastography. Liver stiffness declined from 8.6 to 6.0 kPa one year after surgery. CAP values declined from 322 dB/m at baseline to 251 dB/m at one year. These changes paralleled the histological changes in 32 of the patients[85]. The decrease in liver stiffness was consistent with a decrease from 6.95 to 5.37 kPa in another study of 38 patients after bariatric surgery[92]. Another study of 100 prospectively included bariatric patients reported a decrease from 12.9 to 7.1 kPa[87].
Magnetic resonance imaging (MRI) is increasingly used in the assessment of liver pathology. A prospective study of 31 obese patients undergoing RYGB demonstrated a significant reduction in hepatic fat content using MRI spectroscopy 12 mo after surgery[93]. A decrease in the liver fat fraction using MRI was also observed six[94] and 12 mo[95] after surgery in other studies. These results were supported by a decreased or complete resolution of liver steatosis/NAFLD on ultrasonography one to five years after SG[96,97]. A decrease in hepatic left lobe volume on ultrasound was also observed after two years in 75 women who underwent laparoscopic AGB[98]. Two small studies found improvements in liver damage and NAFLD using ultrasound imaging[99] and CT[100].
Additional exploratory results include decreased serum levels of the hepatocyte apoptosis marker cytokeratin (CK)-18 one year after surgery in nine patients, which was maintained approximately four years later with corresponding improvements in liver histology[81]. Significant improvements in metabolic liver function capacity using LiMAx, which is based on hepatic 13C-methacetin metabolism by cytochrome P450 1A2, were also observed six and 12 mo after surgery[101].
Overall, current studies reported no significant effects on liver-related mortality after bariatric surgery, but larger long-term follow-up studies are necessary to firmly establish the effect of bariatric surgery on liver-related mortality. A recent nation-wide study of patients after bariatric surgery observed no increase in all-cause mortality compared with the general population. However, there was an increased mortality rate ratio 2.01 (95%CI: 1.06-3.84) for gastrointestinal and liver diseases, including peritonitis and intestinal obstruction[102]. Mortality was significantly reduced in patients undergoing bariatric surgery compared to a propensity-score matched cohort of obese patients, but there was no difference in survival when the analysis was restricted to include NASH patients only[103]. Liver cirrhosis is a relative contraindication to bariatric surgery. However, no increased risks of postoperative complications or cirrhosis-related complications were observed in 13 cirrhosis patients undergoing SG with a follow-up of 18 mo. Weight loss in the cirrhosis patients was comparable to the non-cirrhotic patients[104].
In conclusion, bariatric surgery has the potential to induce great weight loss and improve the features of metabolic syndrome and T2D. Recent data demonstrate significant effects of bariatric surgery on GLP-1 and other gut hormones and important lipid metabolic and inflammatory abnormalities involved in the pathophysiology of NAFLD. Therefore, bariatric surgery may reverse the pathological liver changes in NAFLD and NASH patients. Several cohort studies demonstrated improvements in NASH histology, but some studies reported worsened liver histology after bariatric surgery. No studies demonstrated reduced liver-related mortality.
Large randomized clinical trials with long-term follow-up are needed to demonstrate the beneficial effects of bariatric surgery and identify a definitive role of bariatric surgery in NASH patients.
| 1. | Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 919] [Article Influence: 70.7] [Reference Citation Analysis (2)] |
| 2. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2651] [Article Influence: 189.4] [Reference Citation Analysis (3)] |
| 3. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2325] [Article Influence: 155.0] [Reference Citation Analysis (1)] |
| 4. | Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524-530.e1; quiz e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 796] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 5. | Cortez-Pinto H, Camilo ME, Baptista A, De Oliveira AG, De Moura MC. Non-alcoholic fatty liver: another feature of the metabolic syndrome? Clin Nutr. 1999;18:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 6. | Prashanth M, Ganesh HK, Vima MV, John M, Bandgar T, Joshi SR, Shah SR, Rathi PM, Joshi AS, Thakkar H, Menon PS, Shah NS. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Assoc Physicians India. 2009;57:205-210. [PubMed] |
| 7. | Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, Cassader M, David E, Cavallo-Perin P, Rizzetto M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 544] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 8. | Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 1531] [Article Influence: 61.2] [Reference Citation Analysis (7)] |
| 9. | Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014;2:152-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 10. | Lassailly G, Caiazzo R, Buob D, Pigeyre M, Verkindt H, Labreuche J, Raverdy V, Leteurtre E, Dharancy S, Louvet A, Romon M, Duhamel A, Pattou F, Mathurin P. Bariatric Surgery Reduces Features of Nonalcoholic Steatohepatitis in Morbidly Obese Patients. Gastroenterology. 2015;149:379-388; quiz e15-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 573] [Article Influence: 52.1] [Reference Citation Analysis (4)] |
| 11. | Maciejewski ML, Arterburn DE, Van Scoyoc L, Smith VA, Yancy WS, Weidenbacher HJ, Livingston EH, Olsen MK. Bariatric Surgery and Long-term Durability of Weight Loss. JAMA Surg. 2016;151:1046-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 535] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 12. | Brunt EM, Wong VW, Nobili V, Day CP, Sookoian S, Maher JJ, Bugianesi E, Sirlin CB, Neuschwander-Tetri BA, Rinella ME. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1:15080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 635] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 13. | Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2701] [Cited by in RCA: 2698] [Article Influence: 149.9] [Reference Citation Analysis (2)] |
| 14. | Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjærg-Hansen A, Vogt TF, Hobbs HH, Cohen JC. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352-356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 970] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 15. | Pirola CJ, Gianotti TF, Burgueño AL, Rey-Funes M, Loidl CF, Mallardi P, Martino JS, Castaño GO, Sookoian S. Epigenetic modification of liver mitochondrial DNA is associated with histological severity of nonalcoholic fatty liver disease. Gut. 2013;62:1356-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 16. | Sookoian S, Rosselli MS, Gemma C, Burgueño AL, Fernández Gianotti T, Castaño GO, Pirola CJ. Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: impact of liver methylation of the peroxisome proliferator-activated receptor γ coactivator 1α promoter. Hepatology. 2010;52:1992-2000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 17. | Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2112] [Cited by in RCA: 2672] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 18. | Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 780] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 19. | Cusi K. Role of insulin resistance and lipotoxicity in non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:545-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (1)] |
| 20. | Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 956] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 21. | Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 840] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 22. | Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferré P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 599] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 23. | Jacome-Sosa MM, Parks EJ. Fatty acid sources and their fluxes as they contribute to plasma triglyceride concentrations and fatty liver in humans. Curr Opin Lipidol. 2014;25:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 798] [Article Influence: 42.0] [Reference Citation Analysis (2)] |
| 25. | Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52:774-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 868] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 26. | Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147:765-783.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 596] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 27. | Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 812] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 28. | Tosello-Trampont AC, Landes SG, Nguyen V, Novobrantseva TI, Hahn YS. Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. J Biol Chem. 2012;287:40161-40172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 363] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 29. | Wehr A, Baeck C, Ulmer F, Gassler N, Hittatiya K, Luedde T, Neumann UP, Trautwein C, Tacke F. Pharmacological inhibition of the chemokine CXCL16 diminishes liver macrophage infiltration and steatohepatitis in chronic hepatic injury. PLoS One. 2014;9:e112327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1361] [Cited by in RCA: 1604] [Article Influence: 84.4] [Reference Citation Analysis (1)] |
| 31. | Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5073] [Cited by in RCA: 4761] [Article Influence: 216.4] [Reference Citation Analysis (2)] |
| 32. | Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1305] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 33. | Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR; STAMPEDE Investigators. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376:641-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1626] [Cited by in RCA: 2016] [Article Influence: 224.0] [Reference Citation Analysis (0)] |
| 34. | Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1340] [Cited by in RCA: 1307] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 35. | Leonetti F, Capoccia D, Coccia F, Casella G, Baglio G, Paradiso F, Abbatini F, Iossa A, Soricelli E, Basso N. Obesity, type 2 diabetes mellitus, and other comorbidities: a prospective cohort study of laparoscopic sleeve gastrectomy vs medical treatment. Arch Surg. 2012;147:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Ackerman NB. Observations on the improvements in carbohydrate metabolism in diabetic and other morbidly obese patients after jejunoileal bypass. Surg Gynecol Obstet. 1981;152:581-586. [PubMed] |
| 37. | Bojsen-Møller KN, Dirksen C, Jørgensen NB, Jacobsen SH, Serup AK, Albers PH, Hansen DL, Worm D, Naver L, Kristiansen VB, Wojtaszewski JF, Kiens B, Holst JJ, Richter EA, Madsbad S. Early enhancements of hepatic and later of peripheral insulin sensitivity combined with increased postprandial insulin secretion contribute to improved glycemic control after Roux-en-Y gastric bypass. Diabetes. 2014;63:1725-1737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 38. | Jørgensen NB, Jacobsen SH, Dirksen C, Bojsen-Møller KN, Naver L, Hvolris L, Clausen TR, Wulff BS, Worm D, Lindqvist Hansen D, Madsbad S, Holst JJ. Acute and long-term effects of Roux-en-Y gastric bypass on glucose metabolism in subjects with Type 2 diabetes and normal glucose tolerance. Am J Physiol Endocrinol Metab. 2012;303:E122-E131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 256] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 39. | Jørgensen NB, Dirksen C, Bojsen-Møller KN, Jacobsen SH, Worm D, Hansen DL, Kristiansen VB, Naver L, Madsbad S, Holst JJ. Exaggerated glucagon-like peptide 1 response is important for improved β-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes. 2013;62:3044-3052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 231] [Cited by in RCA: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 40. | Romero F, Nicolau J, Flores L, Casamitjana R, Ibarzabal A, Lacy A, Vidal J. Comparable early changes in gastrointestinal hormones after sleeve gastrectomy and Roux-En-Y gastric bypass surgery for morbidly obese type 2 diabetic subjects. Surg Endosc. 2012;26:2231-2239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 41. | Dirksen C, Bojsen-Møller KN, Jørgensen NB, Jacobsen SH, Kristiansen VB, Naver LS, Hansen DL, Worm D, Holst JJ, Madsbad S. Exaggerated release and preserved insulinotropic action of glucagon-like peptide-1 underlie insulin hypersecretion in glucose-tolerant individuals after Roux-en-Y gastric bypass. Diabetologia. 2013;56:2679-2687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Falkén Y, Hellström PM, Holst JJ, Näslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96:2227-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 43. | Nguyen NQ, Debreceni TL, Bambrick JE, Bellon M, Wishart J, Standfield S, Rayner CK, Horowitz M. Rapid gastric and intestinal transit is a major determinant of changes in blood glucose, intestinal hormones, glucose absorption and postprandial symptoms after gastric bypass. Obesity (Silver Spring). 2014;22:2003-2009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Melissas J, Leventi A, Klinaki I, Perisinakis K, Koukouraki S, de Bree E, Karkavitsas N. Alterations of global gastrointestinal motility after sleeve gastrectomy: a prospective study. Ann Surg. 2013;258:976-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 45. | Kuhre RE, Christiansen CB, Saltiel MY, Wewer Albrechtsen NJ, Holst JJ. On the relationship between glucose absorption and glucose-stimulated secretion of GLP-1, neurotensin, and PYY from different intestinal segments in the rat. Physiol Rep. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 46. | Jorsal T, Rhee NA, Pedersen J, Wahlgren CD, Mortensen B, Jepsen SL, Jelsing J, Dalbøge LS, Vilmann P, Hassan H, Hendel JW, Poulsen SS, Holst JJ, Vilsbøll T, Knop FK. Enteroendocrine K and L cells in healthy and type 2 diabetic individuals. Diabetologia. 2018;61:284-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 47. | Svegliati-Baroni G, Saccomanno S, Rychlicki C, Agostinelli L, De Minicis S, Candelaresi C, Faraci G, Pacetti D, Vivarelli M, Nicolini D, Garelli P, Casini A, Manco M, Mingrone G, Risaliti A, Frega GN, Benedetti A, Gastaldelli A. Glucagon-like peptide-1 receptor activation stimulates hepatic lipid oxidation and restores hepatic signalling alteration induced by a high-fat diet in nonalcoholic steatohepatitis. Liver Int. 2011;31:1285-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 333] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 48. | Vetter ML, Wadden TA, Teff KL, Khan ZF, Carvajal R, Ritter S, Moore RH, Chittams JL, Iagnocco A, Murayama K, Korus G, Williams NN, Rickels MR. GLP-1 plays a limited role in improved glycemia shortly after Roux-en-Y gastric bypass: a comparison with intensive lifestyle modification. Diabetes. 2015;64:434-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 49. | Steven S, Hollingsworth KG, Small PK, Woodcock SA, Pucci A, Aribasala B, Al-Mrabeh A, Batterham RL, Taylor R. Calorie restriction and not glucagon-like peptide-1 explains the acute improvement in glucose control after gastric bypass in Type 2 diabetes. Diabet Med. 2016;33:1723-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 50. | Kotronen A, Vehkavaara S, Seppälä-Lindroos A, Bergholm R, Yki-Järvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab. 2007;293:E1709-E1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 169] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 51. | Jackness C, Karmally W, Febres G, Conwell IM, Ahmed L, Bessler M, McMahon DJ, Korner J. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell Function in type 2 diabetic patients. Diabetes. 2013;62:3027-3032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 52. | Jiménez A, Mari A, Casamitjana R, Lacy A, Ferrannini E, Vidal J. GLP-1 and glucose tolerance after sleeve gastrectomy in morbidly obese subjects with type 2 diabetes. Diabetes. 2014;63:3372-3377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Salehi M, Vella A, McLaughlin T, Patti ME. Hypoglycemia After Gastric Bypass Surgery: Current Concepts and Controversies. J Clin Endocrinol Metab. 2018;103:2815-2826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 54. | Dirksen C, Eiken A, Bojsen-Møller KN, Svane MS, Martinussen C, Jørgensen NB, Holst JJ, Madsbad S. No Islet Cell Hyperfunction, but Altered Gut-Islet Regulation and Postprandial Hypoglycemia in Glucose-Tolerant Patients 3 Years After Gastric Bypass Surgery. Obes Surg. 2016;26:2263-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 55. | Salehi M, Gastaldelli A, D'Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology. 2014;146:669-680.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 56. | Knop FK, Taylor R. Mechanism of metabolic advantages after bariatric surgery: it's all gastrointestinal factors versus it's all food restriction. Diabetes Care. 2013;36 Suppl 2:S287-S291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 57. | Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, Torres-Gonzalez A, Gra-Oramas B, Gonzalez-Fabian L, Friedman SL, Diago M, Romero-Gomez M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology. 2015;149:367-378.e5; quiz e14-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1775] [Article Influence: 161.4] [Reference Citation Analysis (3)] |
| 58. | Yousseif A, Emmanuel J, Karra E, Millet Q, Elkalaawy M, Jenkinson AD, Hashemi M, Adamo M, Finer N, Fiennes AG, Withers DJ, Batterham RL. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes Surg. 2014;24:241-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 205] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 59. | Laurenius A, Larsson I, Bueter M, Melanson KJ, Bosaeus I, Forslund HB, Lönroth H, Fändriks L, Olbers T. Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes (Lond). 2012;36:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 60. | le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lönroth H, Fändriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg. 2007;246:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 564] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 61. | Svane MS, Jørgensen NB, Bojsen-Møller KN, Dirksen C, Nielsen S, Kristiansen VB, Toräng S, Wewer Albrechtsen NJ, Rehfeld JF, Hartmann B, Madsbad S, Holst JJ. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int J Obes (Lond). 2016;40:1699-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 62. | Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med. 2002;346:1623-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1709] [Cited by in RCA: 1546] [Article Influence: 64.4] [Reference Citation Analysis (0)] |
| 63. | Faraj M, Havel PJ, Phélis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 348] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 64. | Patti ME, Houten SM, Bianco AC, Bernier R, Larsen PR, Holst JJ, Badman MK, Maratos-Flier E, Mun EC, Pihlajamaki J, Auwerx J, Goldfine AB. Serum bile acids are higher in humans with prior gastric bypass: potential contribution to improved glucose and lipid metabolism. Obesity (Silver Spring). 2009;17:1671-1677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 504] [Cited by in RCA: 462] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 65. | Palleja A, Kashani A, Allin KH, Nielsen T, Zhang C, Li Y, Brach T, Liang S, Feng Q, Jørgensen NB, Bojsen-Møller KN, Dirksen C, Burgdorf KS, Holst JJ, Madsbad S, Wang J, Pedersen O, Hansen T, Arumugam M. Roux-en-Y gastric bypass surgery of morbidly obese patients induces swift and persistent changes of the individual gut microbiota. Genome Med. 2016;8:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 234] [Article Influence: 23.4] [Reference Citation Analysis (1)] |
| 66. | Klein S, Mittendorfer B, Eagon JC, Patterson B, Grant L, Feirt N, Seki E, Brenner D, Korenblat K, McCrea J. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1564-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 67. | Viana EC, Araujo-Dasilio KL, Miguel GP, Bressan J, Lemos EM, Moyses MR, de Abreu GR, de Azevedo JL, Carvalho PS, Passos-Bueno MR, Errera FI, Bissoli NS. Gastric bypass and sleeve gastrectomy: the same impact on IL-6 and TNF-α. Prospective clinical trial. Obes Surg. 2013;23:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 68. | Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, Mendez-Sanchez N, Lizardi-Cervera J, Uribe M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010;CD007340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (1)] |
| 69. | Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2008;6:1396-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 357] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 70. | Kral JG, Thung SN, Biron S, Hould FS, Lebel S, Marceau S, Simard S, Marceau P. Effects of surgical treatment of the metabolic syndrome on liver fibrosis and cirrhosis. Surgery. 2004;135:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 230] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 71. | Stratopoulos C, Papakonstantinou A, Terzis I, Spiliadi C, Dimitriades G, Komesidou V, Kitsanta P, Argyrakos T, Hadjiyannakis E. Changes in liver histology accompanying massive weight loss after gastroplasty for morbid obesity. Obes Surg. 2005;15:1154-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 72. | Mathurin P, Gonzalez F, Kerdraon O, Leteurtre E, Arnalsteen L, Hollebecque A, Louvet A, Dharancy S, Cocq P, Jany T, Boitard J, Deltenre P, Romon M, Pattou F. The evolution of severe steatosis after bariatric surgery is related to insulin resistance. Gastroenterology. 2006;130:1617-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 123] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 73. | Mathurin P, Hollebecque A, Arnalsteen L, Buob D, Leteurtre E, Caiazzo R, Pigeyre M, Verkindt H, Dharancy S, Louvet A, Romon M, Pattou F. Prospective study of the long-term effects of bariatric surgery on liver injury in patients without advanced disease. Gastroenterology. 2009;137:532-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 363] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 74. | Caiazzo R, Lassailly G, Leteurtre E, Baud G, Verkindt H, Raverdy V, Buob D, Pigeyre M, Mathurin P, Pattou F. Roux-en-Y gastric bypass versus adjustable gastric banding to reduce nonalcoholic fatty liver disease: a 5-year controlled longitudinal study. Ann Surg. 2014;260:893-898; discussion 898-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 184] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 75. | Taitano AA, Markow M, Finan JE, Wheeler DE, Gonzalvo JP, Murr MM. Bariatric surgery improves histological features of nonalcoholic fatty liver disease and liver fibrosis. J Gastrointest Surg. 2015;19:429-436; discussion 436-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 76. | Weiner RA. Surgical treatment of non-alcoholic steatohepatitis and non-alcoholic fatty liver disease. Dig Dis. 2010;28:274-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 77. | Moretto M, Kupski C, da Silva VD, Padoin AV, Mottin CC. Effect of bariatric surgery on liver fibrosis. Obes Surg. 2012;22:1044-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 78. | Vargas V, Allende H, Lecube A, Salcedo MT, Baena-Fustegueras JA, Fort JM, Rivero J, Ferrer R, Catalán R, Pardina E, Ramón Y Cajal S, Guardia J, Peinado-Onsurbe J. Surgically induced weight loss by gastric bypass improves non alcoholic fatty liver disease in morbid obese patients. World J Hepatol. 2012;4:382-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 79. | Tai CM, Huang CK, Hwang JC, Chiang H, Chang CY, Lee CT, Yu ML, Lin JT. Improvement of nonalcoholic fatty liver disease after bariatric surgery in morbidly obese Chinese patients. Obes Surg. 2012;22:1016-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 80. | Praveen Raj P, Gomes RM, Kumar S, Senthilnathan P, Karthikeyan P, Shankar A, Palanivelu C. The effect of surgically induced weight loss on nonalcoholic fatty liver disease in morbidly obese Indians: "NASHOST" prospective observational trial. Surg Obes Relat Dis. 2015;11:1315-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 81. | Schneck AS, Anty R, Patouraux S, Bonnafous S, Rousseau D, Lebeaupin C, Bailly-Maitre B, Sans A, Tran A, Gugenheim J, Iannelli A, Gual P. Roux-En Y Gastric Bypass Results in Long-Term Remission of Hepatocyte Apoptosis and Hepatic Histological Features of Non-alcoholic Steatohepatitis. Front Physiol. 2016;7:344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 82. | Froylich D, Corcelles R, Daigle C, Boules M, Brethauer S, Schauer P. Effect of Roux-en-Y gastric bypass and sleeve gastrectomy on nonalcoholic fatty liver disease: a comparative study. Surg Obes Relat Dis. 2016;12:127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 83. | Aldoheyan T, Hassanain M, Al-Mulhim A, Al-Sabhan A, Al-Amro S, Bamehriz F, Al-Khalidi H. The effects of bariatric surgeries on nonalcoholic fatty liver disease. Surg Endosc. 2017;31:1142-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 84. | Manco M, Mosca A, De Peppo F, Caccamo R, Cutrera R, Giordano U, De Stefanis C, Alisi A, Baumann U, Silecchia G, Nobili V. The Benefit of Sleeve Gastrectomy in Obese Adolescents on Nonalcoholic Steatohepatitis and Hepatic Fibrosis. J Pediatr. 2017;180:31-37.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 85. | Garg H, Aggarwal S, Shalimar, Yadav R, Datta Gupta S, Agarwal L, Agarwal S. Utility of transient elastography (fibroscan) and impact of bariatric surgery on nonalcoholic fatty liver disease (NAFLD) in morbidly obese patients. Surg Obes Relat Dis. 2018;14:81-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 86. | Aguilar-Olivos NE, Almeda-Valdes P, Aguilar-Salinas CA, Uribe M, Méndez-Sánchez N. The role of bariatric surgery in the management of nonalcoholic fatty liver disease and metabolic syndrome. Metabolism. 2016;65:1196-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 87. | Nickel F, Tapking C, Benner L, Sollors J, Billeter AT, Kenngott HG, Bokhary L, Schmid M, von Frankenberg M, Fischer L, Mueller S, Müller-Stich BP. Bariatric Surgery as an Efficient Treatment for Non-Alcoholic Fatty Liver Disease in a Prospective Study with 1-Year Follow-up : BariScan Study. Obes Surg. 2018;28:1342-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 88. | Cazzo E, Jimenez LS, Pareja JC, Chaim EA. Effect of Roux-en-Y gastric bypass on nonalcoholic fatty liver disease evaluated through NAFLD fibrosis score: a prospective study. Obes Surg. 2015;25:982-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 89. | Jimenez LS, Mendonça Chaim FH, Mendonça Chaim FD, Utrini MP, Gestic MA, Chaim EA, Cazzo E. Impact of Weight Regain on the Evolution of Non-alcoholic Fatty Liver Disease After Roux-en-Y Gastric Bypass: a 3-Year Follow-up. Obes Surg. 2018;28:3131-3135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 90. | Loy JJ, Youn HA, Schwack B, Kurian M, Ren Fielding C, Fielding GA. Improvement in nonalcoholic fatty liver disease and metabolic syndrome in adolescents undergoing bariatric surgery. Surg Obes Relat Dis. 2015;11:442-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 91. | Simo KA, McKillop IH, McMillan MT, Ahrens WA, Walters AL, Thompson KJ, Kuwada TS, Martinie JB, Iannitti DA, Gersin KS, Sindram D. Does a calculated "NAFLD fibrosis score" reliably negate the need for liver biopsy in patients undergoing bariatric surgery? Obes Surg. 2014;24:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 92. | Naveau S, Lamouri K, Pourcher G, Njiké-Nakseu M, Ferretti S, Courie R, Tranchart H, Ghinoiu M, Balian A, Prévot S, Perlemuter G, Dagher I. The diagnostic accuracy of transient elastography for the diagnosis of liver fibrosis in bariatric surgery candidates with suspected NAFLD. Obes Surg. 2014;24:1693-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 93. | Fjeldborg K, Pedersen SB, Møller HJ, Richelsen B. Reduction in serum fibroblast growth factor-21 after gastric bypass is related to changes in hepatic fat content. Surg Obes Relat Dis. 2017;13:1515-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 94. | Hedderich DM, Hasenberg T, Haneder S, Schoenberg SO, Kücükoglu Ö, Canbay A, Otto M. Effects of Bariatric Surgery on Non-alcoholic Fatty Liver Disease: Magnetic Resonance Imaging Is an Effective, Non-invasive Method to Evaluate Changes in the Liver Fat Fraction. Obes Surg. 2017;27:1755-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 95. | Jiménez-Agüero R, Emparanza JI, Beguiristain A, Bujanda L, Alustiza JM, García E, Hijona E, Gallego L, Sánchez-González J, Perugorria MJ, Asensio JI, Larburu S, Garmendia M, Larzabal M, Portillo MP, Aguirre L, Banales JM. Novel equation to determine the hepatic triglyceride concentration in humans by MRI: diagnosis and monitoring of NAFLD in obese patients before and after bariatric surgery. BMC Med. 2014;12:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | Ruiz-Tovar J, Alsina ME, Alpera MR; OBELCHE Group. Improvement of nonalcoholic fatty liver disease in morbidly obese patients after sleeve gastrectomy: association of ultrasonographic findings with lipid profile and liver enzymes. Acta Chir Belg. 2017;117:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 97. | Algooneh A, Almazeedi S, Al-Sabah S, Ahmed M, Othman F. Non-alcoholic fatty liver disease resolution following sleeve gastrectomy. Surg Endosc. 2016;30:1983-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 98. | Giannetti M, Piaggi P, Ceccarini G, Mazzeo S, Querci G, Fierabracci P, Salvetti G, Galli G, Ricco I, Martinelli S, Di Salvo C, Anselmino M, Landi A, Vitti P, Pinchera A, Santini F. Hepatic left lobe volume is a sensitive index of metabolic improvement in obese women after gastric banding. Int J Obes (Lond). 2012;36:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 99. | Major P, Pędziwiatr M, Rubinkiewicz M, Stanek M, Głuszewska A, Pisarska M, Małczak P, Budzyński A, Budzyński P. Impact of bariatric surgery on non-alcoholic fatty liver disease. Pol Przegl Chir. 2017;89:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 100. | Winder JS, Dudeck BS, Schock S, Lyn-Sue JR, Haluck RS, Rogers AM. Radiographic Improvement of Hepatic Steatosis After Laparoscopic Roux-en-Y Gastric Bypass. Obes Surg. 2017;27:376-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 101. | Alizai PH, Wendl J, Roeth AA, Klink CD, Luedde T, Steinhoff I, Neumann UP, Schmeding M, Ulmer F. Functional Liver Recovery After Bariatric Surgery--a Prospective Cohort Study with the LiMAx Test. Obes Surg. 2015;25:2047-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 102. | Gribsholt SB, Thomsen RW, Svensson E, Richelsen B. Overall and cause-specific mortality after Roux-en-Y gastric bypass surgery: A nationwide cohort study. Surg Obes Relat Dis. 2017;13:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 103. | Goossens N, Hoshida Y, Song WM, Jung M, Morel P, Nakagawa S, Zhang B, Frossard JL, Spahr L, Friedman SL, Negro F, Rubbia-Brandt L, Giostra E. Nonalcoholic Steatohepatitis Is Associated With Increased Mortality in Obese Patients Undergoing Bariatric Surgery. Clin Gastroenterol Hepatol. 2016;14:1619-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 104. | Rebibo L, Gerin O, Verhaeghe P, Dhahri A, Cosse C, Regimbeau JM. Laparoscopic sleeve gastrectomy in patients with NASH-related cirrhosis: a case-matched study. Surg Obes Relat Dis. 2014;10:405-10; quiz 565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Denmark
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Alam S, Deneau M, Jamali R, Marciano S S- Editor: Ji FF L- Editor: A E- Editor: Zhang YL