修回日期: 2024-03-20
接受日期: 2024-04-15
在线出版日期: 2024-05-28
胃内菌群与胃癌(gastric cancer, GC)的关系是近年来的研究热点之一. 研究发现胃内菌群通过多种途径参与GC发生, 且在肿瘤发展过程中出现特征性改变. 幽门螺杆菌被认为是Ⅰ类致癌原, 在GC发展中发挥重要作用. 近年来, 越来越多研究发现非幽门螺杆菌同样参与GC发展. 本文通过检索知网、PubMed、Web of Science, 围绕胃内微生态与GC的相互关系、作用机制及临床价值展开综述, 以期为GC的临床诊疗提供新思路.
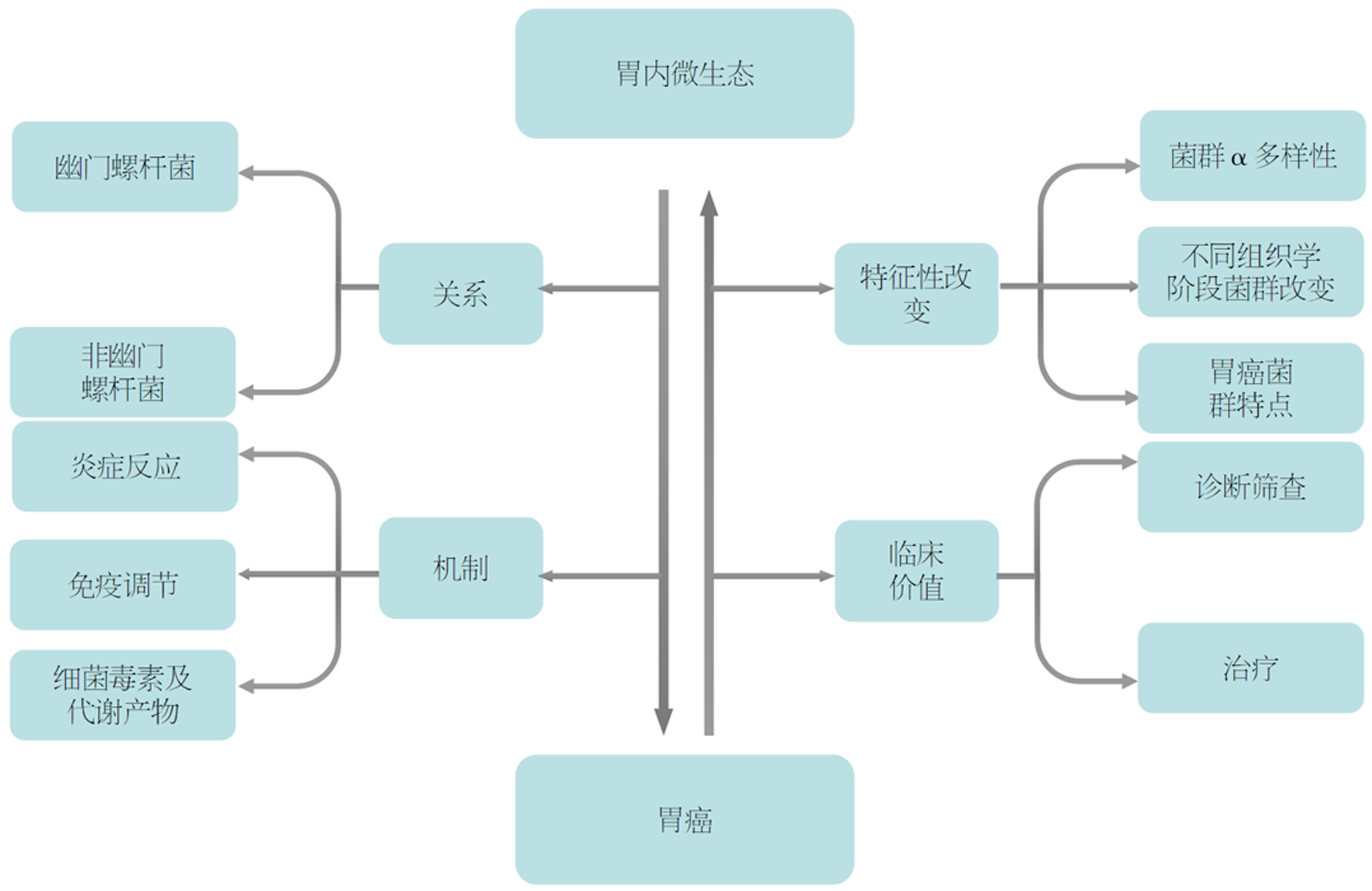
核心提要: 胃内微生态通过炎症、免疫、毒素及代谢产物等途径参与胃癌发生. 在Correa级联中, 不同组织学阶段胃内菌群发生显著改变. 随着胃癌发展, 胃粘膜菌群与胃液菌群差异逐渐减小, 且胃癌阶段出现了特征性菌群富集.
引文著录: 王锦丽, 靖大道. 胃内微生态与胃癌: 关系、机制与临床价值. 世界华人消化杂志 2024; 32(5): 327-332
Revised: March 20, 2024
Accepted: April 15, 2024
Published online: May 28, 2024
The relationship between gastric microbiome and gastric cancer has been one of the research focuses in recent years. Many studies have found that gastric microbiome is involved in the occurrence of tumor through several ways, and appears to have characteristic changes in the development of gastric cancer. Helicobacter pylori (H. pylori) is considered a class I carcinogen, which plays an important role in the development of gastric cancer. More studies have recently discovered that bacteria other than H. pylori are also responsible for the growth of gastric cancer. In this review, by searching CNKI, PubMed, and Web of Science, we provide an overview on the connection, mechanism, and clinical significance between gastric microbiome and gastric cancer, with an aim to offer fresh perspectives on the diagnosis and treatment of gastric cancer.
- Citation: Wang JL, Jing DD. Gastric microbiome and gastric cancer: Relationship, mechanism, and clinical significance. Shijie Huaren Xiaohua Zazhi 2024; 32(5): 327-332
- URL: https://www.wjgnet.com/1009-3079/full/v32/i5/327.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v32.i5.327
胃癌(gastric cancer, GC)是一种常见的消化系统恶性肿瘤, 具有高发病率和高死亡率的特点[1]. 目前认为, GC的危险因素包括幽门螺杆菌(Helicobacter pylori, H. pylori)感染、年龄、家族史、吸烟、饮酒、高盐摄入、水果和蔬菜摄入少等[2]. 近年来, 越来越多研究表明[3-5], 胃内微生态与GC的发展密切相关. 本文就胃内微生态与GC发生、发展的相互关系, 促癌机制及炎癌转变中胃内微生态变化进行简要综述, 并阐述胃内菌群在GC诊疗中的临床价值, 以期为GC临床诊疗提供新思路.
目前认为, 胃内具有独特的微生态系统, 在门水平上主要由放线菌门、拟杆菌门、厚壁菌门、梭杆菌门和变形菌门构成[6]. 近年来, 越来越多研究表明[3-5], 胃内微生物群与GC的发生发展密切相关. 在一项探究韩国人群胃内微生物群相对丰度和GC风险关系的病例对照研究中, Gunathilake等[7]发现H. pylori相对丰度高、痤疮丙酸杆菌相对丰度高以及携带粪便普雷沃氏菌的人群有更高的GC风险(OR值分别为1.86, 4.77, 2.54). Lertpiriyapong等[8]发现H. pylori与肠道菌群共定植加速了胰岛素-胃泌素转基因鼠胃上皮内瘤变的进展.与无菌小鼠、限制性改良后Schaedler菌群(restricted Altered Schaedler's flora, rASF)定植小鼠、单H. pylori定植小鼠、肠道菌群定植小鼠相比, H. pylori与肠道菌群共定植小鼠或H. pylori与rASF共定植小鼠具有更严重的胃黏膜病变. H. pylori感染与GC密切相关, 被国际癌症研究机构归类为I类致癌原[9]. 但也有学者提出, GC进展似乎与H. pylori感染无关.一方面, 胃腺癌的微生物群主要由肠道和口腔菌群组成. 另一方面, 即便成功根除H. pylori也无法影响GC进展[10]. 有学者认为, H. pylori刺激慢性萎缩性胃炎的发展, 而非直接诱导胃癌产生. 胃黏膜萎缩导致胃内酸度下降, 进一步影响胃微生物群的构成. 这种菌群变化在GC发生后期可能发挥更重要作用, 诱导慢性萎缩性胃炎向肠化生发展, 最终发展为GC[11].
微生物群落通过募集免疫细胞, 分泌炎症介质, 激活细胞内信号通路等途径调控炎症反应, 加速肿瘤的生长. H. pylori被认为是胃黏膜炎症发生的重要因素, 能促进中性粒细胞募集浸润至胃黏膜, 加剧炎症反应[12]. H. pylori感染可以引起多种炎症介质释放, 包括白细胞介素-8(interleukin-8, IL-8)、巨噬细胞趋化蛋白-1、白细胞介素-1β等[13]. Dang等[14]发现H. pylori通过Toll样受体2激活核因子κB, 进而调控p53上调凋亡调控因子, 促进胃炎及癌症进展. Zuo等[15]发现H. pylori还可以激活WNT/β-catenin信号通路, 诱导胃炎发展. 其它胃内菌群同样能促进炎症反应, 甚至诱导GC发生. Sung等[16]发现, 鲁氏不动杆菌、咽峡炎链球菌和罗尔斯通氏菌与胃内慢性炎症相关. Miyata等[17]发现微黄奈瑟菌能显著刺激胃黏膜细胞分泌IL-8, 使炎症持续存在, 并增加GC风险. 痤疮丙酸杆菌是一种经典的皮肤细菌, 近期也被确认为胃内微生物, 其可引起淋巴细胞性胃炎, 通过产生促炎细胞因子如白细胞介素-15, 促进GC发展[7].
微生物群落通过免疫识别、免疫逃逸、调节免疫应答等途径促进肿瘤发生发展. 研究[18]发现, 胃上皮细胞作为先天性免疫应答的第一道防线, 其表达模式识别受体如Toll样受体(toll-like receptors, TLRs), TLRs通过识别不同细菌成分(如脂多糖、肽聚糖等)激活细胞信号级联, 在适应性免疫应答和宿主炎症反应中发挥重要作用. Ling等[19]发现胃内非优势菌群如寡养单胞菌属、月形单胞菌属、丛毛单胞菌属等胃微生物群与浆细胞样树突状细胞和调节性T细胞显著相关, 二者是重要的免疫抑制细胞, 在肿瘤免疫逃逸中起关键作用. Holokai等[20]发现, H. pylori感染后通过Sonic hedgehog (Shh)信号通路介导程序性死亡配体1(programmed death ligand 1, PD-L1)在胃上皮细胞表达, 引起免疫应答失调. 球形马拉色菌被认为是与PD-L1表达相关的胃内真菌, 在PD-L1高表达组中显著升高[21]. PD-L1是一种抑制性分子, 可与T细胞的程序性死亡受体-1相互作用, 阻断T细胞增殖和活性. 在肿瘤细胞中, PD-L1表达可使肿瘤细胞逃避宿主抗肿瘤免疫[22]. 还有研究发现[23]PD-L1过表达与肿瘤侵袭相关.
2.3.1 细胞毒素相关蛋白A: 细胞毒素相关蛋白A(cytotoxin-associated protein A, CagA)是H. pylori产生的一种重要毒力因子, 被证实能增加癌症风险[24]. 研究表明[24-26], CagA通过抑制细胞凋亡、促进上皮间充质转化(epithelial-mesenchymal transition, EMT)、诱导DNA损伤和修复缺陷等多种途径促进胃肿瘤发生.
2.3.2 空泡毒素A: 空泡毒素A(vacuolating cytotoxin A, VacA)是一种成孔细胞毒素, 能促进细胞内空泡形成[27]. Capurro等[28]发现, VacA通过靶向溶酶体钙通道瞬时受体电位粘蛋白1, 破坏溶酶体运输和抑制自噬杀伤, 形成利于细菌定植的细胞内生态位. Abdullah等[27]发现, VacA通过破坏自噬促进胃上皮细胞CagA积累. VacA还能靶向胃粘膜中的髓系细胞形成一种耐受性环境, 促进调节性T细胞分化, 抑制效应T细胞功能, 影响免疫应答[29].
2.3.3 幽门螺杆菌肿瘤坏死因子-α诱导蛋白: 幽门螺杆菌肿瘤坏死因子-α诱导蛋白是H. pylori分泌的一种毒力因子, 其与胃上皮细胞表面的核仁素结合进入细胞质, 诱导TNF-α和其他促炎细胞因子的产生. 其还与EMT相关, 在癌症中发挥重要作用[30,31].
2.3.4 短链脂肪酸: 短链脂肪酸(short-chain fatty acid , SCFA), 如丁酸盐、丙酸盐和乙酸盐, 是菌群代谢产物之一. 丁酸盐和丙酸盐能通过调控细胞周期(G2-M期阻滞)参与诱导GC细胞凋亡[32]. 在一项基于牛瘤胃上皮细胞(bovine rumen epithelial cells, BRECs)的研究中发现, SCFA通过G蛋白偶联受体41调节参与免疫细胞募集和上皮免疫屏障的基因水平, 进而调控BRECs的先天性免疫应答[33].
2.3.5 脲酶: 脲酶是H. pylori产生的一种毒力因子, 能分解尿素产生氨, 中和胃内酸性环境, 还能促进细菌定植在胃上皮细胞, 并诱导产生强烈炎症反应. 另外, 脲酶能诱导人胃腺癌细胞中促血管生成因子表达, 抑制抗血管生成因子表达, 参与GC发展[34]. 另有研究认为[35], 脲酶通过介导激活中性粒细胞和血小板, 在胃炎中发挥重要作用.
2.3.6 N-亚硝基化合物: 在GC进程中, 胃内环境改变促进产N-亚硝基化合物(N-nitroso compounds, NOC)细菌定植. NOC能刺激原癌基因的表达, 诱导血管生成和抑制细胞凋亡[36]. NOC还可直接作用于DNA, 诱导DNA突变和双链断裂, 参与肿瘤的形成和发展[37].
2.3.7 乳酸: 研究发现[38], 乳酸通过MCT-HIF1α信号通路促进巨噬细胞极化至M2样状态. M2巨噬细胞可以通过分泌转化生长因子-β、血管内皮生长因子等参与EMT和血管生成, 促进肿瘤细胞的侵袭和转移. 此外, 乳酸还能通过Akt信号途径增强高迁移率族蛋白盒1磷酸化, 促进其核转位及释放, 进而参与诱导GC细胞增殖和迁移[39].
Correa级联是目前被广泛接受的GC发病模式, 认为GC的发展是从正常胃黏膜-非萎缩性胃炎-萎缩性胃炎(atrophic gastritis, AG)-肠上皮化生(intestinal metaplasia, IM)-异型增生(dysplasia, Dys)-GC逐渐发展的过程[40]. 目前多数研究认为[9,41], 胃内微生态在胃癌炎癌转变中发生显著变化. Sun等[9]运用16S rRNA基因测序, 在H. pylori阴性患者中, 对浅表性胃炎(superficial gastritis, SG), AG, IM, Dys和GC患者的胃粘膜进行分析, 采用Shannon和Chao 1指数描述菌群的α多样性, 发现从SG, AG, IM, Dys到GC, 随着胃黏膜病变进展, 微生物群落的多样性和丰富度呈下降趋势. 这与Wang、Li等研究的结论一致[41,42]. 也有结论相反的研究[43], 认为菌群的α多样性在正常胃黏膜中最高, 其次是IM组和GC组, SG组和AG组的多样性最小. Zhang等[44]则认为在GC发生的各个阶段, 胃内微生物群的丰富度和多样性无逐渐变化趋势. 这种差异可能是因为: (1)H. pylori定植对胃微生物群的组成和多样性有显著影响[45]; (2)胃内微生态受饮食、抗生素、长期质子泵抑制剂或H2受体阻滞剂的使用等多种因素影响[46]; (3)不同研究的样本量、研究方法等存在差异.
在GC发展的不同组织学阶段中, Sun等[9]发现, 在H. pylori阴性患者中, 从AG到Dys的癌前病变阶段, 伯克氏菌科丰度持续增加, 而链球菌科和普雷沃氏菌科丰度持续下降. IM组和Dys组的胃粘膜微生物群特征相似, 以罗尔斯通菌属和红球菌属为优势菌属. Pimentel-Nunes等[47]在一项研究正常胃黏膜、萎缩性胃炎伴肠化、早期胃癌(early gastric cancer, EGC)患者的胃黏膜菌群的研究中发现, IM组和EGC组以厚壁菌门为主, 孪生球菌属及链球菌属从对照组到GC组呈逐渐增加趋势, 且在IM阶段已经显著存在生态失调. Wang等[41]发现IM组与慢性非萎缩性胃炎组的胃微生物群具有相似性, 上皮内瘤变组(intraepithelial neoplasia, IN)与GC组的胃微生物群具有相似性. 其认为在IM向IN进展过程中, 胃内微生物群可能发生巨大转变, 且癌前病变患者的微生物组成在门水平上已经发生变化. 以上研究表明, 在GC发展的不同组织学阶段, 胃内微生物群发生显著改变, 特别是在IM阶段. IM阶段是否是GC发生的重要节点, 通过胃内微生态改变促进炎癌转变, 这尚待进一步研究.
在GC进程中, 胃液菌群也发生了改变. Wei等[3]发现健康组、早期胃癌组和进展期GC组胃液菌群的多样性和丰度存在差异. He等[48]在一项探究SG, IM, GC患者的胃黏膜及胃液菌群的研究中发现胃黏膜中的微生物组成与胃液显著不同, 且从SG到GC进程中, 这种差异显著缩小. 其进一步探究了导致胃黏膜和胃液菌群差异缩小的特定细菌, 发现在GC患者中产NOC细菌(如韦荣氏球菌属、嗜血杆菌属等)在胃黏膜样本和胃液样本中丰度相近, 而在SG患者中, 胃液样本的菌群丰度显著高于胃黏膜样本. Li等[42]在一项荟萃分析中同样发现, 随着疾病进展, 胃粘膜和胃液菌群之间的差异程度逐渐降低, 这可能与GC中胃黏膜屏障受损, 外源性细菌进行性侵袭相关.
GC患者的菌群特点表现为螺杆菌属减少, 肠道共生菌、口腔菌群和产乳酸菌群的富集[49,50]. Ferreira等[50]发现, 与慢性胃炎患者相比, GC患者的菌群特征表现为微生物多样性减低, 螺杆菌属低丰度及其他菌属富集(主要是肠道共生菌), 且亚硝化细菌被认为是GC患者的特征菌落. Hsieh等[51]在一项分析胃炎、IM、GC组胃上皮微生物群的研究中同样发现H. pylori丰度在GC组显著降低. 研究[50]发现, GC患者中富集的肠道共生菌包括柠檬酸杆菌属、梭菌属、乳杆菌属、无色杆菌属、红球菌属等. 另外, 多项研究表明[4,49,52], GC患者中口腔菌群富集, 包括梭杆菌属、韦荣氏球菌属、纤毛菌属、嗜血杆菌属、弯曲杆菌属、普雷沃氏菌属、链球菌属、消化链球菌属等. Wei等[3]通过分析健康人、早期GC、晚期GC患者的胃液, 发现乳杆菌属是晚期GC组的特征性菌属. 近期的一项荟萃分析[42]同样发现, 与胃炎相比, 乳酸菌如双歧杆菌、乳杆菌属、咽峡炎链球菌在GC患者中显著富集. 但是也有研究表明[52], 与GC组织相比, 产乳酸菌群在邻近的非癌组织中更为富集. 以上研究均提示GC患者的胃内微生态存在特征性变化.
目前, 关于胃内微生态的临床价值方面的研究主要集中在GC的诊断筛查. Liu等[53]通过构建胃黏膜菌群的随机森林模型, 发现该模型在H. pylori阳性患者中区分癌前病变和浅表性胃炎表现出优异的性能(AUC = 0.794). Wang等[41]同样基于5种胃内菌群构建随机森林模型, 该模型能够准确地识别胃组织学类型. He等[48]选择7个胃黏膜菌属和13个胃液菌属分别构建随机森林模型, 该模型能有效区分GC和SG, 且在一个独立队列中得到验证. Hsieh等[51]发现梭菌、梭杆菌和乳杆菌在GC患者中富集, 认为clostridium colicanis和具核梭杆菌可作为诊断GC的生物标记物. 在治疗方面, 近期一项纳入15例研究的荟萃分析发现[54], H. pylori根除治疗可以逆转癌前病变并预防其进展, 但这仅适用于表观遗传学改变和轻度病变的患者. 另外一篇荟萃分析[55]得出同样的结论, 认为早期根除H. pylori可显著改善AG和IM.
综上所述, 胃内微生态在GC的发生发展中发挥重要作用, 胃内菌群特别是非幽门螺杆菌在炎癌转变后期发生了特征性、趋势性改变, 这种改变已越来越被证实通过各种途径参与GC的发生. 关于胃内微生态与GC之间的联系见图1. 在炎癌转变过程中, GC患者的胃黏膜出现了特定菌群富集, 明确这种特征性改变有助于GC的早期诊断. 目前, GC的诊断主要依赖于消化内镜及病理活检, 对于内镜下表现尚不明显的早期肿瘤, 消化内镜和病理活检存在一定的漏诊率. 而胃内菌群联合消化内镜及病理活检或许能成为GC诊断的新方法, 有望提升GC的漏诊率和高危人群的检出率, 从而对其进行早期识别干预. 目前关于胃内微生态的临床价值方面研究相对较少, 未来仍需扩大样本量进一步发掘其潜在的临床价值, 将微生物学的发现转化为有效的诊断治疗策略, 为GC的诊治提供新的临床思路.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [PubMed] [DOI] |
| 2. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [PubMed] [DOI] |
| 3. | Wei Q, Zhang Q, Wu Y, Han S, Yin L, Zhang J, Gao Y, Shen H, Zhuang J, Chu J, Liu J, Wei Y. Analysis of bacterial diversity and community structure in gastric juice of patients with advanced gastric cancer. Discov Oncol. 2023;14. [PubMed] [DOI] |
| 4. | Liu C, Ng SK, Ding Y, Lin Y, Liu W, Wong SH, Sung JJ, Yu J. Meta-analysis of mucosal microbiota reveals universal microbial signatures and dysbiosis in gastric carcinogenesis. Oncogene. 2022;41:3599-3610. [PubMed] [DOI] |
| 5. | Gunathilake M, Lee J, Choi IJ, Kim YI, Yoon J, Sul WJ, Kim JF, Kim J. Alterations in Gastric Microbial Communities Are Associated with Risk of Gastric Cancer in a Korean Population: A Case-Control Study. Cancers (Basel). 2020;12. [PubMed] [DOI] |
| 6. | Bik EM, Eckburg PB, Gill SR, Nelson KE, Purdom EA, Francois F, Perez-Perez G, Blaser MJ, Relman DA. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci USA. 2006;103:732-737. [PubMed] [DOI] |
| 7. | Gunathilake MN, Lee J, Choi IJ, Kim YI, Ahn Y, Park C, Kim J. Association between the relative abundance of gastric microbiota and the risk of gastric cancer: a case-control study. Sci Rep. 2019;9:13589. [PubMed] [DOI] |
| 8. | Lertpiriyapong K, Whary MT, Muthupalani S, Lofgren JL, Gamazon ER, Feng Y, Ge Z, Wang TC, Fox JG. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut. 2014;63:54-63. [PubMed] [DOI] |
| 9. | Sun QH, Zhang J, Shi YY, Zhang J, Fu WW, Ding SG. Microbiome changes in the gastric mucosa and gastric juice in different histological stages of Helicobacter pylori-negative gastric cancers. World J Gastroenterol. 2022;28:365-380. [PubMed] [DOI] |
| 10. | Liatsos C, Papaefthymiou A, Kyriakos N, Galanopoulos M, Doulberis M, Giakoumis M, Petridou E, Mavrogiannis C, Rokkas T, Kountouras J. Helicobacter pylori, gastric microbiota and gastric cancer relationship: Unrolling the tangle. World J Gastrointest Oncol. 2022;14:959-972. [PubMed] [DOI] |
| 11. | Dias-Jácome E, Libânio D, Borges-Canha M, Galaghar A, Pimentel-Nunes P. Gastric microbiota and carcinogenesis: the role of non-Helicobacter pylori bacteria - A systematic review. Rev Esp Enferm Dig. 2016;108:530-540. [PubMed] [DOI] |
| 12. | Lopes C, Almeida TC, Pimentel-Nunes P, Dinis-Ribeiro M, Pereira C. Linking dysbiosis to precancerous stomach through inflammation: Deeper than and beyond imaging. Front Immunol. 2023;14:1134785. [PubMed] [DOI] |
| 13. | Jan I, Rather RA, Mushtaq I, Malik AA, Besina S, Baba AB, Farooq M, Yousuf T, Rah B, Afroze D. Helicobacter pylori Subdues Cytokine Signaling to Alter Mucosal Inflammation via Hypermethylation of Suppressor of Cytokine Signaling 1 Gene During Gastric Carcinogenesis. Front Oncol. 2021;10:604747. [PubMed] [DOI] |
| 14. | Dang Y, Zhang Y, Xu L, Zhou X, Gu Y, Yu J, Jin S, Ji H, Shu Y, Zhang G, Cui S, Sun J. PUMA-mediated epithelial cell apoptosis promotes Helicobacter pylori infection-mediated gastritis. Cell Death Dis. 2020;11:139. [PubMed] [DOI] |
| 15. | Zuo W, Yang H, Li N, Ouyang Y, Xu X, Hong J. Helicobacter pylori infection activates Wnt/β-catenin pathway to promote the occurrence of gastritis by upregulating ASCL1 and AQP5. Cell Death Discov. 2022;8:257. [PubMed] [DOI] |
| 16. | Sung JJY, Coker OO, Chu E, Szeto CH, Luk STY, Lau HCH, Yu J. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut. 2020;69:1572-1580. [PubMed] [DOI] |
| 17. | Miyata N, Hayashi Y, Hayashi S, Sato K, Hirai Y, Yamamoto H, Sugano K. Lipopolysaccharides From Non-Helicobacter pylori Gastric Bacteria Potently Stimulate Interleukin-8 Production in Gastric Epithelial Cells. Clin Transl Gastroenterol. 2019;10:e00024. [PubMed] [DOI] |
| 18. | Eed EM, Hawash YA, Khalifa AS, Alsharif KF, Alghamdi SA, Almalki AA, Almehmadi MM, Ismail KA, Taha AA, Saber T. Association of toll-like receptors 2, 4, 9 and 10 genes polymorphisms and Helicobacter pylori-related gastric diseases in Saudi patients. Indian J Med Microbiol. 2020;38:94-100. [PubMed] [DOI] |
| 19. | Ling Z, Shao L, Liu X, Cheng Y, Yan C, Mei Y, Ji F, Liu X. Regulatory T Cells and Plasmacytoid Dendritic Cells Within the Tumor Microenvironment in Gastric Cancer Are Correlated With Gastric Microbiota Dysbiosis: A Preliminary Study. Front Immunol. 2019;10:533. [PubMed] [DOI] |
| 20. | Holokai L, Chakrabarti J, Broda T, Chang J, Hawkins JA, Sundaram N, Wroblewski LE, Peek RM, Wang J, Helmrath M, Wells JM, Zavros Y. Increased Programmed Death-Ligand 1 is an Early Epithelial Cell Response to Helicobacter pylori Infection. PLoS Pathog. 2019;15:e1007468. [PubMed] [DOI] |
| 21. | Zhang Z, Qiu Y, Feng H, Huang D, Weng B, Xu Z, Xie Q, Wang Z, Ding W, Li G, Liu H. Identification of Malassezia globosa as a Gastric Fungus Associated with PD-L1 Expression and Overall Survival of Patients with Gastric Cancer. J Immunol Res. 2022;2022:2430759. [PubMed] [DOI] |
| 22. | Amirmoezi F, Geramizadeh B. Molecular Classification of Gastric Cancer With Emphasis on PDL-1 Expression: The First Report From Iran. Clin Pathol. 2022;15:2632010X221096378. [PubMed] [DOI] |
| 23. | Oki E, Okano S, Saeki H, Umemoto Y, Teraishi K, Nakaji Y, Ando K, Zaitsu Y, Yamashita N, Sugiyama M, Nakashima Y, Ohgaki K, Oda Y, Maehara Y. Protein Expression of Programmed Death 1 Ligand 1 and HER2 in Gastric Carcinoma. Oncology. 2017;93:387-394. [PubMed] [DOI] |
| 24. | Palrasu M, Zaika E, El-Rifai W, Garcia-Buitrago M, Piazuelo MB, Wilson KT, Peek RM, Zaika AI. Bacterial CagA protein compromises tumor suppressor mechanisms in gastric epithelial cells. J Clin Invest. 2020;130:2422-2434. [PubMed] [DOI] |
| 25. | Shi Y, Yang Z, Zhang T, Shen L, Li Y, Ding S. SIRT1-targeted miR-543 autophagy inhibition and epithelial-mesenchymal transition promotion in Helicobacter pylori CagA-associated gastric cancer. Cell Death Dis. 2019;10:625. [PubMed] [DOI] |
| 26. | Imai S, Ooki T, Murata-Kamiya N, Komura D, Tahmina K, Wu W, Takahashi-Kanemitsu A, Knight CT, Kunita A, Suzuki N, Del Valle AA, Tsuboi M, Hata M, Hayakawa Y, Ohnishi N, Ueda K, Fukayama M, Ushiku T, Ishikawa S, Hatakeyama M. Helicobacter pylori CagA elicits BRCAness to induce genome instability that may underlie bacterial gastric carcinogenesis. Cell Host Microbe. 2021;29:941-958.e10. [PubMed] [DOI] |
| 27. | Abdullah M, Greenfield LK, Bronte-Tinkew D, Capurro MI, Rizzuti D, Jones NL. VacA promotes CagA accumulation in gastric epithelial cells during Helicobacter pylori infection. Sci Rep. 2019;9:38. [PubMed] [DOI] |
| 28. | Capurro MI, Greenfield LK, Prashar A, Xia S, Abdullah M, Wong H, Zhong XZ, Bertaux-Skeirik N, Chakrabarti J, Siddiqui I, O'Brien C, Dong X, Robinson L, Peek RM, Philpott DJ, Zavros Y, Helmrath M, Jones NL. VacA generates a protective intracellular reservoir for Helicobacter pylori that is eliminated by activation of the lysosomal calcium channel TRPML1. Nat Microbiol. 2019;4:1411-1423. [PubMed] [DOI] |
| 29. | Altobelli A, Bauer M, Velez K, Cover TL, Müller A. Helicobacter pylori VacA Targets Myeloid Cells in the Gastric Lamina Propria To Promote Peripherally Induced Regulatory T-Cell Differentiation and Persistent Infection. mBio. 2019;10. [PubMed] [DOI] |
| 30. | Morningstar-Wright L, Czinn SJ, Piazuelo MB, Banerjee A, Godlewska R, Blanchard TG. The TNF-Alpha Inducing Protein is Associated With Gastric Inflammation and Hyperplasia in a Murine Model of Helicobacter pylori Infection. Front Pharmacol. 2022;13:817237. [PubMed] [DOI] |
| 31. | Mahant S, Chakraborty A, Som A, Mehra S, Das K, Mukhopadhyay AK, Gehlot V, Bose S, Das R. The Synergistic Role of Tip α, Nucleolin and Ras in Helicobacter pylori Infection Regulates the Cell Fate Towards Inflammation or Apoptosis. Curr Microbiol. 2021;78:3720-3732. [PubMed] [DOI] |
| 32. | Matthews GM, Howarth GS, Butler RN. Short-chain fatty acid modulation of apoptosis in the Kato III human gastric carcinoma cell line. Cancer Biol Ther. 2007;6:1051-1057. [PubMed] [DOI] |
| 33. | Zhan K, Gong X, Chen Y, Jiang M, Yang T, Zhao G. Short-Chain Fatty Acids Regulate the Immune Responses via G Protein-Coupled Receptor 41 in Bovine Rumen Epithelial Cells. Front Immunol. 2019;10:2042. [PubMed] [DOI] |
| 34. | Olivera-Severo D, Uberti AF, Marques MS, Pinto MT, Gomez-Lazaro M, Figueiredo C, Leite M, Carlini CR. A New Role for Helicobacter pylori Urease: Contributions to Angiogenesis. Front Microbiol. 2017;8:1883. [PubMed] [DOI] |
| 35. | Scopel-Guerra A, Olivera-Severo D, Staniscuaski F, Uberti AF, Callai-Silva N, Jaeger N, Porto BN, Carlini CR. The Impact of Helicobacter pylori Urease upon Platelets and Consequent Contributions to Inflammation. Front Microbiol. 2017;8:2447. [PubMed] [DOI] |
| 36. | Majewski M, Mertowska P, Mertowski S, Smolak K, Grywalska E, Torres K. Microbiota and the Immune System-Actors in the Gastric Cancer Story. Cancers (Basel). 2022;14. [PubMed] [DOI] |
| 37. | Chen YX, He LL, Xiang XP, Shen J, Qi HY. O(6)-methylguanine DNA methyltransferase is upregulated in malignant trans-formation of gastric epithelial cells via its gene promoter DNA hypomethylation. World J Gastrointest Oncol. 2022;14:664-677. [PubMed] [DOI] |
| 38. | Zhang L, Li S. Lactic acid promotes macrophage polarization through MCT-HIF1α signaling in gastric cancer. Exp Cell Res. 2020;388:111846. [PubMed] [DOI] |
| 40. | Sun D, Lei L, Xia C, Li H, Cao M, He S, Zhang Z, Guo G, Song G, Peng J, Chen W. Sociodemographic disparities in gastric cancer and the gastric precancerous cascade: A population-based study. Lancet Reg Health West Pac. 2022;23:100437. [PubMed] [DOI] |
| 41. | Wang Z, Gao X, Zeng R, Wu Q, Sun H, Wu W, Zhang X, Sun G, Yan B, Wu L, Ren R, Guo M, Peng L, Yang Y. Changes of the Gastric Mucosal Microbiome Associated With Histological Stages of Gastric Carcinogenesis. Front Microbiol. 2020;11:997. [PubMed] [DOI] |
| 42. | Li Y, Hu Y, Zhan X, Song Y, Xu M, Wang S, Huang X, Xu ZZ. Meta-analysis reveals Helicobacter pylori mutual exclusivity and reproducible gastric microbiome alterations during gastric carcinoma progression. Gut Microbes. 2023;15:2197835. [PubMed] [DOI] |
| 43. | Gantuya B, El Serag HB, Matsumoto T, Ajami NJ, Uchida T, Oyuntsetseg K, Bolor D, Yamaoka Y. Gastric mucosal microbiota in a Mongolian population with gastric cancer and precursor conditions. Aliment Pharmacol Ther. 2020;51:770-780. [PubMed] [DOI] |
| 44. | Zhang X, Li C, Cao W, Zhang Z. Alterations of Gastric Microbiota in Gastric Cancer and Precancerous Stages. Front Cell Infect Microbiol. 2021;11:559148. [PubMed] [DOI] |
| 45. | Li TH, Qin Y, Sham PC, Lau KS, Chu KM, Leung WK. Alterations in Gastric Microbiota After H. Pylori Eradication and in Different Histological Stages of Gastric Carcinogenesis. Sci Rep. 2017;7:44935. [PubMed] [DOI] |
| 46. | Espinoza JL, Matsumoto A, Tanaka H, Matsumura I. Gastric microbiota: An emerging player in Helicobacter pylori-induced gastric malignancies. Cancer Lett. 2018;414:147-152. [PubMed] [DOI] |
| 47. | Pimentel-Nunes P, Barros A, Pita I, Miranda I, Conceição G, Borges-Canha M, Leite-Moreira AF, Libânio D, Dinis-Ribeiro M. Gastric microbiome profile throughout gastric carcinogenesis: beyond helicobacter. Scand J Gastroenterol. 2021;56:708-716. [PubMed] [DOI] |
| 48. | He C, Peng C, Shu X, Wang H, Zhu Z, Ouyang Y, Yang X, Xie C, Hu Y, Li N, Ge Z, Zhu Y, Lu N. Convergent dysbiosis of gastric mucosa and fluid microbiome during stomach carcinogenesis. Gastric Cancer. 2022;25:837-849. [PubMed] [DOI] |
| 49. | Castaño-Rodríguez N, Goh KL, Fock KM, Mitchell HM, Kaakoush NO. Dysbiosis of the microbiome in gastric carcinogenesis. Sci Rep. 2017;7:15957. [PubMed] [DOI] |
| 50. | Ferreira RM, Pereira-Marques J, Pinto-Ribeiro I, Costa JL, Carneiro F, Machado JC, Figueiredo C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut. 2018;67:226-236. [PubMed] [DOI] |
| 51. | Hsieh YY, Tung SY, Pan HY, Yen CW, Xu HW, Lin YJ, Deng YF, Hsu WT, Wu CS, Li C. Increased Abundance of Clostridium and Fusobacterium in Gastric Microbiota of Patients with Gastric Cancer in Taiwan. Sci Rep. 2018;8:158. [PubMed] [DOI] |
| 52. | Chen XH, Wang A, Chu AN, Gong YH, Yuan Y. Mucosa-Associated Microbiota in Gastric Cancer Tissues Compared With Non-cancer Tissues. Front Microbiol. 2019;10:1261. [PubMed] [DOI] |
| 53. | Liu D, Chen S, Gou Y, Yu W, Zhou H, Zhang R, Wang J, Ye F, Liu Y, Sun B, Zhang K. Gastrointestinal Microbiota Changes in Patients With Gastric Precancerous Lesions. Front Cell Infect Microbiol. 2021;11:749207. [PubMed] [DOI] |
| 54. | Zhu F, Zhang X, Li P, Zhu Y. Effect of Helicobacter pylori eradication on gastric precancerous lesions: A systematic review and meta-analysis. Helicobacter. 2023;28:e13013. [PubMed] [DOI] |
| 55. | Liang Y, Yang Y, Nong R, Huang H, Chen X, Deng Y, Huang Z, Huang J, Cheng C, Ji M, Chen Y, Hu F. Do atrophic gastritis and intestinal metaplasia reverse after Helicobacter pylori eradication? Helicobacter. 2024;29:e13042. [PubMed] [DOI] |
学科分类: 胃肠病学和肝病学
手稿来源地: 上海市
同行评议报告学术质量分类
A级 (优秀): 0
B级 (非常好): B, B
C级 (良好): C
D级 (一般): 0
E级 (差): 0
科学编辑: 张砚梁 制作编辑:张砚梁