修回日期: 2024-09-24
接受日期: 2024-10-30
在线出版日期: 2024-11-28
肝细胞癌(hepatocellular carcinoma, HCC)是最常见的癌症之一. HCC起病隐匿, 多数患者首次诊断时已丧失根治性手术的机会, 系统性的抗肿瘤治疗成为中晚期HCC治疗的关键. 其中抗肿瘤药物出现耐药性是HCC疗效不佳, 影响HCC患者预后的重要原因之一, 如何改善HCC的治疗效果仍是现今研究的重点. 尽管国内外对以新生血管生成为基础的抗肿瘤药物的研究不断深入, 但对共用正常组织血管来满足肿瘤自身代谢需求的血管共选择(vascular co-option)模式研究较少, 其对HCC的进展及抗肿瘤治疗的影响也未被人考虑在内. 本文就血管co-option对HCC多种治疗方式的影响及相关机制进行概述, 以期为改善HCC耐药奠定理论基础.
核心提要: 肝癌病程进展迅速, 大部分肝癌发现时已经处于晚期阶段, 高达80%的患者确诊时即丧失局部治疗的机会. 如何有效抑制肝癌的血液供应, 改善相关药物耐药, 并探明相关机制仍是一个难题. 重视肿瘤血管co-option这一不依赖新生血管生成的肿瘤供血方式有望成为抗肿瘤治疗的突破点.
引文著录: 齐明皓, 李景涛, 翟博. 重视血管co-option在肝癌治疗中的潜在机制及治疗靶点作用. 世界华人消化杂志 2024; 32(11): 827-834
Revised: September 24, 2024
Accepted: October 30, 2024
Published online: November 28, 2024
Hepatocellular carcinoma (HCC) is one of the most common cancers, which has an insidious onset, and most of the patients have already lost the chance of radical surgery at the time of the first diagnosis, so systematic antitumor therapy has become the key to the treatment of intermediate and advanced HCC. The emergence of drug resistance to antitumor drugs is one of the most important reasons for the poor efficacy, which affects the prognosis of HCC patients, and how to improve the therapeutic efficacy for HCC is still the main focus of the present research. Although the research on antitumor drugs based on neovascularization has been deepening both domestically and abroad, less research has been done on the vascular co-option mode, which shares blood vessels of normal tissues to meet the metabolic needs of the tumor itself, and its impact on the progression of HCC and antitumor therapy has not been extensively studied. In this paper, we provide an overview of the impact of vascular co-option on multiple treatment modalities for hepatocellular carcinoma and related mechanisms, with a view to laying a theoretical foundation for improving drug resistance in HCC.
- Citation: Qi MH, Li JT, Zhai B. Mechanisms of vascular co-option as a potential therapeutic target in hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi 2024; 32(11): 827-834
- URL: https://www.wjgnet.com/1009-3079/full/v32/i11/827.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v32.i11.827
肝细胞癌(hepatocellular carcinoma, HCC)在世界范围内位居肿瘤相关死亡原因的第3位[1]. 在我国因各种不同病因导致的肝癌增长正持续加快, 年均新发病例和死亡病例占全球发病一半以上, 死亡率达13.94%[2]. 现有的HCC治疗手段主要包括局部手术治疗、系统抗肿瘤治疗、化疗、放射治疗等, 手术切除被认为是最佳的治疗方法[3,4]. 然而, 因HCC起病隐匿, 大多数患者确诊时已处于中晚期阶段, 仅有少数患者可接受根治性手术治疗, 且手术后HCC的复发和转移很常见[1]. 通过系统性抗肿瘤治疗控制中晚期HCC患者疾病进展, 已成为HCC治疗的关键.
现有靶向治疗药物如仑伐替尼, 索拉非尼等多激酶抑制剂效果有限, 延长患者的生存期尚不足3个月[5]. 其主要通过靶向血管内皮生长因子受体(vascular endothelial growth factor receptor, VEGFR), 血小板衍生的生长因子受体(platelet-derived growth factor receptor, PDGFR)、干细胞因子受体(mast/stem cell growth factor receptor, KIT)、成纤维细胞生长因子受体(fibroblast growth factor receptor, FGFR)、酪氨酸蛋白激酶-2(tyrosine kinase-2, Tie2)等多个酪氨酸激酶发挥作用以抑制新生血管生成延长晚期HCC的总生存期, 但仍有35%-43%的患者治疗后出现获得性耐药导致预后不良[6]. 阿替利珠单抗联合贝伐珠单抗、信迪利单抗联合贝伐珠单抗类似物作为一线抗肿瘤药物可明显延长HCC患者中位生存时间和无进展生存期, 但对部分患者的疗效仍然有限[7-10]. 现有研究表明[11,12], 血管co-option可能是影响多种抗肿瘤治疗疗效的原因. 本文将基于血管co-option这一不同于新生血管生成的肿瘤供血方式系统阐述其介导HCC对抗肿瘤药物耐药的潜在机制及研究进展.
血管co-option是肿瘤细胞直接利用正常组织中预先存在的血管系统来满足自身代谢需求的一种新的肿瘤供血方式[13]. 在血管co-option的形成过程中, 癌细胞沿着正常血管的表面进行迁移和/或浸润正常血管之间的组织空间, 最终使肿瘤周围的正常血管并入肿瘤内部. 通过这种方式, 肿瘤可以选择已有的正常血管来满足其自身的代谢需求, 而无需刺激新血管生长[14]. 肿瘤血管co-option保留了良好的血管结构, 这与新生血管生成的无序和混乱的血管结构形成了鲜明的对比[15]. 这一新的肿瘤血管结构及肿瘤供血方式影响了多种抗肿瘤治疗的治疗效果, 促使HCC对现有抗肿瘤药物耐药.
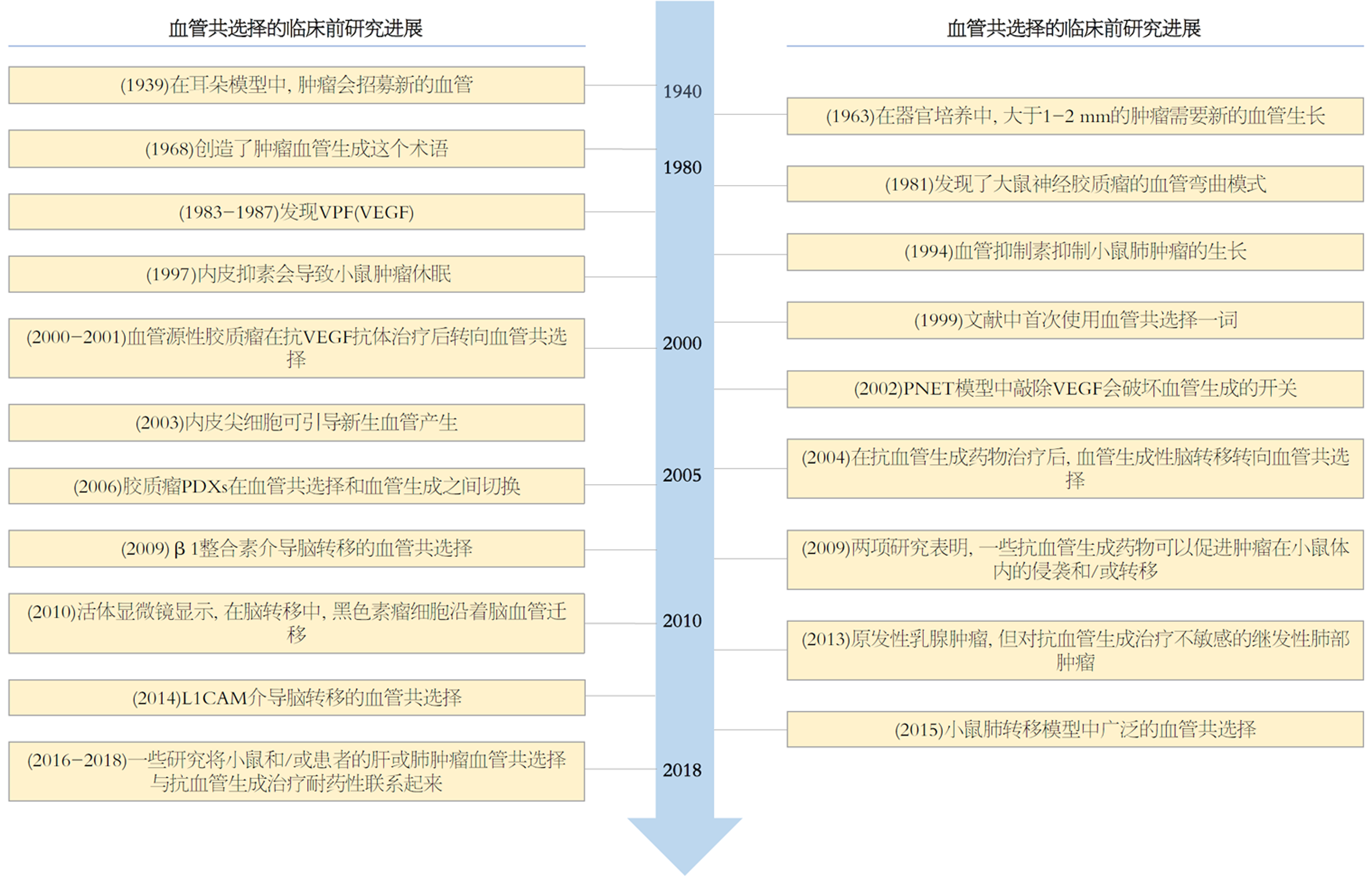
肿瘤依赖于新生血管形成(angiogenesis)的理论最早由 Judah Folkman于1971年提出, 并奠定了当今肿瘤分子靶向治疗的基础[16]. 目前血管生成抑制剂已被广泛用于多种组织来源的恶性肿瘤[17]. 其中治疗HCC的分子靶向药物就主要为血管生成抑制剂, 它们在抑制VEGFR同时还作用于如PDGFR、KIT、FGFR、TIE2等多个酪氨酸激酶[18-20]. 但值得深究的是血管生成抑制剂并未达到预期的理想效果, 其不能长期发挥作用, 治疗过程中又不可避免的会出现疾病进展, 甚至有研究发现抗血管生成治疗可以促进肿瘤的侵袭能力、增加肿瘤的转移率[21,22]. 事实上, 在肿瘤发生发展中, 除了依赖新生血管生成以外, 尚存在血管共选择(Vascular co-option)和血管生成拟态(Vasculogenic mimicry)两种不依赖新生血管生成的机制[23]. 研究文献已经提供了证据表明肿瘤能够通过血管生成或血管co-option(或两者同时)的方式获取血液供应[24-26]. 然而, 尽管已有大量关于血管生成和血管co-option的文献发表, 但后者却往往被忽视, 使得血管生成被视为主要的肿瘤血管形成机制(图1). 由于该领域的共识和认知仍然有限, 这可能阻碍了将血管co-option作为普遍肿瘤血管化机制的广泛接受. 因此, 正确认识血管co-option, 并开发更有效的治疗策略, 将有希望更好地抑制肿瘤的生长和扩散.
抗血管生成治疗的耐药性可分为原发性耐药(治疗开始时即观察到的)和获得性(初步治疗后观察到的)两种. 在抗血管生成药物的Ⅲ期临床试验结果公布之前, 已有研究表明, 依赖血管co-option的肿瘤可能对抗血管生成治疗表现出耐药性[25,27-30]. 在2022版原发性HCC诊疗指南中, 五种抗血管生成药物已获批治疗HCC: 多纳非尼、索拉非尼、仑伐替尼用于晚期HCC的一线治疗, 阿帕替尼和瑞戈非尼用于晚期HCC的二线治疗[4]. 使用获批的抗血管生成药物治疗晚期HCC可适当提高HCC中位生存时间和无进展生存期, 降低患者死亡及疾病进展风险, 但仍有相当一部分患者在疾病治疗过程中出现获得性耐药, 导致药物疗效逐渐降低[31,32]. 抗血管生成药物的疗效一般与一些肿瘤不依赖于新生血管生成, 而通过非血管生成机制利用血液系统供血有关, 其中血管co-option发挥重要作用.
原发性HCC疾病进展过程中可呈现出不同组织病理学生长模式包括与新生血管生成相关的包膜/结缔组织生长模式、与血管co-option相关的替代生长模式等[33-41]. 在肝脏中, 动脉或门静脉的血液供应与肿瘤的血管化模式密切相关. 原发和继发性肝脏肿瘤可能通过未配对的动脉分支形成新血管, 也可能会吸收原有的窦状毛细血管或门静脉血管.
直径小于2厘米的HCC通常表现出两种不同的生长模式: 模糊结节型HCC具有不规则边界, 可以侵犯肝实质(主要利用窦状血管: 一种血管co-option形式); 明显结节型的小进展型HCC可能侵犯门静脉束间质或纤维间隔(主要利用新血管生成)[14,42].
近几年的数据表明, 在原发性HCC中, 血管co-option可能是部分HCC患者对抗血管生成治疗出现先天性和获得性耐药的重要原因[14,25,43,44]. 随着研究的不断深入, 部分研究人员在HCC原位小鼠模型的肿瘤组织中发现了新生血管和正常的肝血管(窦状血管和门脉血管)[45]. 通过对小鼠HCC原位HCC模型使用索拉非尼治疗后可见肿瘤组织新生血管减少, 但肿瘤内部的窦状血管和门脉血管数量也显著增加[45]. 随着治疗时间的延长, 小鼠逐渐产生获得性耐药, 且获得性耐药的增加程度与HCC侵袭的增加程度相一致[44,45]. 在停止索拉非尼治疗后, 耐药HCC将重新转变为利用新生血管生成供血[44,45]. 这表明耐药HCC中的新生血管生成向血管co-option的转变是可逆的; 血管生成和血管co-option之间可以相互转化或同时存在. 以上现象均表明HCC的肿瘤组织依赖血管co-option而非新生血管生成促进肿瘤侵袭, 但血管co-option如何促进HCC的侵袭和转移, 其具体机制尚不明确.
现已证实, HCC的侵袭性表型与HCC细胞中的波形蛋白、E盒结合锌指蛋白1/2(简称Zeb1/2)的表达上调及钙黏蛋白E(E-cadherin, 简称E-cad)的表达下调相关, 这可能是HCC细胞转移促进血管co-option形成的基础[42,44-48]. 因此, 在HCC治疗过程中为防止由血管co-option介导的获得性耐药, 可能有必要将抗血管生成治疗与靶向肿瘤侵袭性的药物相结合.
综上所述, HCC血管co-option与抗血管生成治疗的先天性和获得性耐药性有关. 在继发性肝肿瘤(包括胆管癌和乳腺癌、肺癌、胃癌和黑色素瘤的肝转移)中虽观察到与血管co-option相关的替代生长模式[11,42,49], 但尚未有人研究不同的转移性肝肿瘤中肿瘤生长模式与抗血管生成治疗反应之间的关系.
随着人们对肿瘤免疫疗法理论研究的不断深入、对检查点抑制剂的不断研发, 如何提高人体自身对肿瘤的免疫反应成为抗肿瘤治疗的焦点. 原发性HCC诊疗指南(2022版)指出阿替利珠单抗联合贝伐珠单抗、信迪利单抗联合贝伐珠单抗类似物可用于原发性HCC的一线免疫治疗, 卡瑞利珠单抗、替雷利珠单抗可用于原发性HCC的二线免疫治疗[4]. HCC组织内部存在新生血管生成、血管co-option及血管拟态等多种供血方式, 不同供血方式对HCC的肿瘤免疫微环境的影响各不相同[50,51].
新生血管生成的血管结构与血管co-option的结构相比更加紊乱、无序, 也具有更高的通透性; 新生血管生成会使肿瘤血液灌注受损, 导致肿瘤微环境有缺氧、免疫抑制、治疗抵抗、促进肿瘤转移扩散的倾向[42,52-54]. 部分研究认为血管通透性增高是原发性肿瘤转移和侵袭的关键特征, 通过抗血管生成联合免疫治疗可以促进新生血管正常化, 使更多的效应T细胞到达特定位置发挥抗肿瘤作用, 并通过减少活化的巨噬细胞、骨髓抑制细胞和调节剂T细胞间接改变肿瘤的免疫抑制[2,52,55-58]. 尽管血管正常化本身对HCC的生长没有影响, 但改善血管屏障功能和肿瘤灌注可减少临床前肿瘤模型中HCC的转移和侵袭[1,50,55,59-61]. 靶向VEGF的多激酶抑制剂及单克隆抗体联合免疫治疗对HCC的影响成为现有研究的主体[2,6,61,62], 而基于血管co-option对肿瘤免疫的影响的研究却很少.
血管co-option与正常血管结构相比未有明显不同, 因此无法通过促进血管正常化的方式抑制血管co-option[14,42,54]. 现有研究表明依赖血管co-option的转移性HCC比依赖新生血管生成的转移性HCC免疫细胞浸润的更少, 肿瘤的免疫抑制作用更强[33,37,49]. 同时, 有证据表明效应T细胞浸润水平有限的肿瘤通常对免疫检查点抑制剂的内在抵抗力越强[63-66]. 因此, 可以推测, 依赖血管co-option的肿瘤对免疫检查点抑制剂反应性较低, 血管co-option抑制免疫治疗药物的疗效. 目前, 血管co-option与肿瘤免疫浸润减少之间的相关机制尚不清楚, 但这可能与以下几点原因有关: (1)正常脉管系统通常可抑制异常炎症反应, co-option血管的内皮细胞本质上与正常血管内皮细胞相同[35,62,67,68]. 因此与血管生成的血管内皮细胞相比, co-option血管的免疫功能更"正常"; (2)肿瘤新生血管可能更容易促进肿瘤微环境慢性炎症的发生, 现有研究也已证明血管生成和炎症之间的联系[67]; (3)与血管增生、结缔组织增生转移相比, 血管co-option肝转移通常具有较低的免疫和/或炎症细胞浸润, 肿瘤的免疫浸润有限导致免疫"cold"肿瘤乃至肿瘤"免疫荒漠"的形成, 这可能是肿瘤对免疫治疗耐药的原因[52,69].
目前尚未有研究证明VEGF的表达在新生血管生成肿瘤和血管co-option肿瘤之间有不同[42,70]. 基于肿瘤免疫微环境中VEGF对效应T细胞和抗原提呈细胞的免疫抑制作用[56,62]; 对调节性T细胞、骨髓源性抑制细胞增强作用[52], 原则上通过抑制VEGF可以增强新生血管生成和血管co-option肿瘤的免疫反应, 但这可能与肿瘤血管机制对肿瘤免疫微环境的影响相矛盾. 此外, 仍然缺乏预测性生物标志物去准确识别哪些患者可能从抗血管生成治疗中获益. 目前为止, 尚未有研究直接证实肿瘤血管形成机制对免疫治疗药物(包括程序性细胞死亡受体1/程序性死亡配体1抗体、细胞毒性T淋巴细胞相关抗原4抗体)的影响. 有学者认为, 在转移性HCC中通过血管重塑疗法, 即抗新生血管生成和抗co-option血管联合, 可使新生血管生成正常化同时减少肿瘤对正常血管的粘附作用. 通过联合治疗将改善的肿瘤灌注, 促进淋巴细胞的流入并减轻免疫抑制, 从而创造免疫"中间"环境(介于免疫"cold"肿瘤和免疫"hot"肿瘤之间), 为使用免疫检查点抑制剂激活局部适应性免疫并产生免疫"hot"肿瘤奠定基础[50,69]. 但这一观点在原发性HCC中是否可行仍需进一步证实.
综上所述, 通过判断原发性HCC及其他多种肿瘤的血管形成机制, 进而明确血管co-option肿瘤对免疫治疗的敏感性, 探明血管co-option影响肿瘤免疫的相关机制, 将为肿瘤免疫治疗奠定基础. 探究抗新生血管生成联合抗co-option血管对HCC免疫治疗的影响, 以及单独使用VEGF抑制剂对co-option肿瘤免疫微环境的影响仍十分必要.
经动脉化疗栓塞、放射治疗作为HCC常用的非手术治疗方法[4,71-73], 影响HCC化疗、放疗的因素很多[74], 其中血管co-option对肿瘤血管co-option的HCC化疗、放疗影响仍存在争议.
因HCC血管co-option的形成需要HCC细胞浸润在正常血管周围, 且内皮源性因子可促使临近癌细胞对化疗耐药, 因此有人认为血管co-option可促使HCC对化疗耐药[75]. 此外, 由于肝脏既依赖门静脉供血又依赖肝动脉供血[76], 这一独特的供血方式很可能使依赖血管co-option供血的HCC细胞在经动脉化疗栓塞术后存活, 这也可能是HCC经动脉化疗栓塞术后疗效不佳的原因.
但在一些情况下, 血管co-option可能会提高化疗对HCC的疗效. 由于HCC组织中的co-option血管来源于正常组织, 其血管结构良好、血液供应充足, 因此我们可以推测出全身性化疗药物在部分血管co-option依赖性HCC中可能分布的更广泛, HCC对化疗也更敏感性(这与新生血管生成性肿瘤不同). 同时, 血管co-option依赖性HCC的缺氧生物标志物水平较低[77], 这也间接表明血管co-option的血液供应可能更丰富. 在缺氧肿瘤中放疗治疗的治疗效果不佳, 而co-option血管依赖性肿瘤正好因血供丰富克服了这一点. 因此, 我们也不难推断出血管co-option依赖性肿瘤可能对放疗更敏感.
通过抗血管生成可以促进HCC新生血管正常化, 这可能会促进化疗药物向HCC内递送, 同时增加放疗的疗效[3,78,79]. 但是在前文我们已经提出, 现有的抗血管生成治疗可促进血管co-option的形成, 但由于HCC在肝脏的位置不同, 其血管co-option的来源(来源于门静脉和/或肝动脉)也不同, 这给接受放疗和/或化疗的患者带来的影响, 尚未可知.
血管co-option依赖性HCC通过替代生长模式向周围组织浸润, 这可能会影响手术治疗的过程中肿瘤边缘界限的确定, 从而增加原位复发的风险[80]. 研究发现, 在转移性肝肿瘤的患者手术标本中存在替代生长模式, 这一现象与肝切除术后肿瘤复发风险增加和疾病进展风险增加相关[81,82].
新生血管生成不是HCC进展所必须的, HCC既可以单独使用血管生成, 又可以单独使用血管co-option, 这两种机制可在HCC中同时存在并进行动态切换[42]. 因此为防止血管co-option介导的HCC获得性耐药, 我们有必要抑制新生血管生成时联合抗肿瘤侵袭药物治疗HCC, 以达到延长患者总生存期及无进展生存时间的目的. 血管co-option的形成与肿瘤细胞的运动和侵袭相关, 因此癌细胞运动的相关靶点将有可能成为抑制血管co-option的关键. 研究表示, 通过靶向Arp2/3(一种参与肌动蛋白介导的细胞运动的蛋白质)的表达可抑制血管co-option, 使HCC组织由依赖血管co-option转变为依赖新生血管生成. 在临床前模型中, 同时靶向VEGF和Arp2/3, 可以阻断新生血管生成和癌细胞运动, 控制肿瘤进展[11]. 此外, 通过抑制L1CAM(L1细胞粘附分子)和β1-整合素也有助于抑制血管co-option, 阻止肿瘤向体内多个器官的转移[83-85]. 贝伐单抗与β1-整合素拮抗剂(OS2966)联合可在肿瘤耐药后产生持久的抗肿瘤反应[84].
总之, 血管co-option可能成为是癌症治疗的新靶点, 通过抗血管co-option联合抗血管生成治疗对改善HCC预后, 逆转HCC耐药具有巨大的潜力. 血管co-option对抗肿瘤治疗耐药性的影响正在成为肿瘤治疗研究的一个重要领域.
| 1. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. [PubMed] [DOI] |
| 3. | Hindson J. Combined TACE and sorafenib for HCC treatment. Nat Rev Gastroenterol Hepatol. 2020;17:66. [PubMed] [DOI] |
| 6. | Chan LK, Ng IO. Joining the dots for better liver cancer treatment. Nat Rev Gastroenterol Hepatol. 2020;17:74-75. [PubMed] [DOI] |
| 7. | Dual Immunotherapy Makes Strides against HCC. Cancer Discov. 2022;12:OF1. [PubMed] [DOI] |
| 8. | Yang Z, Zhang X, Bai X, Xi X, Liu W, Zhong W. Anti-angiogenesis in colorectal cancer therapy. Cancer Sci. 2024;115:734-751. [PubMed] [DOI] |
| 9. | Yang D, Dang S, Wang Z, Xie M, Li X, Ding X. Vessel co-option: a unique vascular-immune niche in liver cancer. Front Oncol. 2024;14:1386772. [PubMed] [DOI] |
| 10. | Wei Q, Zhang YH. Flavonoids with Anti-Angiogenesis Function in Cancer. Molecules. 2024;29. [PubMed] [DOI] |
| 11. | Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, Nathan M, Wotherspoon A, Gao ZH, Shi Y, Van den Eynden G, Daley F, Peckitt C, Tan X, Salman A, Lazaris A, Gazinska P, Berg TJ, Eltahir Z, Ritsma L, Van Rheenen J, Khashper A, Brown G, Nystrom H, Sund M, Van Laere S, Loyer E, Dirix L, Cunningham D, Metrakos P, Reynolds AR. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med. 2016;22:1294-1302. [PubMed] [DOI] |
| 12. | Carrera-Aguado I, Marcos-Zazo L, Carrancio-Salán P, Guerra-Paes E, Sánchez-Juanes F, Muñoz-Félix JM. The Inhibition of Vessel Co-Option as an Emerging Strategy for Cancer Therapy. Int J Mol Sci. 2024;25. [PubMed] [DOI] |
| 13. | Kuczynski EA, Reynolds AR. Vessel co-option and resistance to anti-angiogenic therapy. Angiogenesis. 2020;23:55-74. [PubMed] [DOI] |
| 14. | Dudley AC. Introduction to special issue: vascular co-option in cancer. Angiogenesis. 2020;23:1-2. [PubMed] [DOI] |
| 15. | Shao Y, Lu B. The emerging roles of circular RNAs in vessel co-option and vasculogenic mimicry: clinical insights for anti-angiogenic therapy in cancers. Cancer Metastasis Rev. 2022;41:173-191. [PubMed] [DOI] |
| 16. | Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273-286. [PubMed] [DOI] |
| 17. | Si R, Hai P, Zheng Y, Liu N, Wang J, Zhang Q, Li Y, Pan X, Zhang J. Discovery of novel PROTACs based on multi-targeted angiogenesis inhibitors. Bioorg Med Chem Lett. 2023;87:129275. [PubMed] [DOI] |
| 18. | Zhang YL, Cui XJ, Xing H, Ning HF, Dong P, Wang GZ. Molecular targeted therapy and immunotherapy in advanced hepatocellular carcinoma: a systematic review and Bayesian network meta-analysis based on randomized controlled trials. Ann Med. 2023;55:2242384. [PubMed] [DOI] |
| 19. | Wang Y, Deng B. Hepatocellular carcinoma: molecular mechanism, targeted therapy, and biomarkers. Cancer Metastasis Rev. 2023;42:629-652. [PubMed] [DOI] |
| 20. | Griffioen AW, Dudley AC. The rising impact of angiogenesis research. Angiogenesis. 2022;25:435-437. [PubMed] [DOI] |
| 21. | Motta-Guerrero R, Recondo G, Cardona A, Corrales L, Arnao V, Failoc-Rojas VE, Aliaga C. The role of angiogenesis inhibitors associated with tyrosine kinase inhibitors in the first-line treatment for EGFR-mutated advanced lung cancer. Crit Rev Oncol Hematol. 2024;196:104294. [PubMed] [DOI] |
| 22. | Tu J, Liang H, Li C, Huang Y, Wang Z, Chen X, Yuan X. The application and research progress of anti-angiogenesis therapy in tumor immunotherapy. Front Immunol. 2023;14:1198972. [PubMed] [DOI] |
| 23. | Du DD, Gong FL, Zhang WY, Yu BF, Guo XL. Anti-angiogenic Therapy for Tumor: Tumor Angiogenesis, Vasculogenic Mimicry and Vascular Normalization. Journal of Modern Medical Oncology. 2023;3. [DOI] |
| 24. | Cannell IG, Sawicka K, Pearsall I, Wild SA, Deighton L, Pearsall SM, Lerda G, Joud F, Khan S, Bruna A, Simpson KL, Mulvey CM, Nugent F, Qosaj F, Bressan D; CRUK IMAXT Grand Challenge Team, Dive C, Caldas C, Hannon GJ. FOXC2 promotes vasculogenic mimicry and resistance to anti-angiogenic therapy. Cell Rep. 2023;42:112791. [PubMed] [DOI] |
| 25. | Pezzella F, Ribatti D. Vascular co-option and vasculogenic mimicry mediate resistance to antiangiogenic strategies. Cancer Rep (Hoboken). 2022;5:e1318. [PubMed] [DOI] |
| 26. | Cuypers A, Truong AK, Becker LM, Saavedra-García P, Carmeliet P. Tumor vessel co-option: The past & the future. Front Oncol. 2022;12:965277. [PubMed] [DOI] |
| 27. | García-Gómez P, Valiente M. Vascular co-option in brain metastasis. Angiogenesis. 2020;23:3-8. [PubMed] [DOI] |
| 28. | Ribatti D, Annese T, Tamma R. Vascular co-option in resistance to anti-angiogenic therapy. Front Oncol. 2023;13:1323350. [PubMed] [DOI] |
| 29. | Pezzella F, Qian CN. Editorial: Vascular co-option and beyond for cancer biology. Front Oncol. 2023;13:1227540. [PubMed] [DOI] |
| 30. | Pellerino A, Bruno F, Soffietti R, Rudà R. Antiangiogenic Therapy for Malignant Brain Tumors: Does It Still Matter? Curr Oncol Rep. 2023;25:777-785. [PubMed] [DOI] |
| 31. | Ott PA, Hodi FS, Kaufman HL, Wigginton JM, Wolchok JD. Combination immunotherapy: a road map. J Immunother Cancer. 2017;5:16. [PubMed] [DOI] |
| 32. | Vogel A, Martinelli E; ESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol. 2021;32:801-805. [PubMed] [DOI] |
| 33. | van Dam PJ, Daelemans S, Ross E, Waumans Y, Van Laere S, Latacz E, Van Steen R, De Pooter C, Kockx M, Dirix L, Vermeulen PB. Histopathological growth patterns as a candidate biomarker for immunomodulatory therapy. Semin Cancer Biol. 2018;52:86-93. [PubMed] [DOI] |
| 34. | Latacz E, van Dam PJ, Vanhove C, Llado L, Descamps B, Ruiz N, Joye I, Grünhagen D, Van Laere S, Dirix P, Mollevi DG, Verhoef C, Dirix L, Vermeulen P. Can medical imaging identify the histopathological growth patterns of liver metastases? Semin Cancer Biol. 2021;71:33-41. [PubMed] [DOI] |
| 35. | Haas G, Fan S, Ghadimi M, De Oliveira T, Conradi LC. Different Forms of Tumor Vascularization and Their Clinical Implications Focusing on Vessel Co-option in Colorectal Cancer Liver Metastases. Front Cell Dev Biol. 2021;9:612774. [PubMed] [DOI] |
| 36. | Starmans MPA, Buisman FE, Renckens M, Willemssen FEJA, van der Voort SR, Groot Koerkamp B, Grünhagen DJ, Niessen WJ, Vermeulen PB, Verhoef C, Visser JJ, Klein S. Distinguishing pure histopathological growth patterns of colorectal liver metastases on CT using deep learning and radiomics: a pilot study. Clin Exp Metastasis. 2021;38:483-494. [PubMed] [DOI] |
| 37. | Messaoudi N, Henault D, Stephen D, Cousineau I, Simoneau E, Rong Z, Létourneau R, Plasse M, Dagenais M, Roy A, Lapointe R, Vandenbroucke-Menu F, Kunda R, Ysebaert D, Soucy G, Stagg J, Vermeulen P, Turcotte S. Prognostic implications of adaptive immune features in MMR-proficient colorectal liver metastases classified by histopathological growth patterns. Br J Cancer. 2022;126:1329-1338. [PubMed] [DOI] |
| 38. | Nierop PM, Höppener DJ, Buisman FE, van der Stok EP, Galjart B, Balachandran VP, Jarnagin WR, Kingham TP, Shia J, Mauer M, Nordlinger B, Julié C, Groot Koerkamp B, Doukas M, Vermeulen PB, Grünhagen DJ, D'Angelica MI, Verhoef C. Preoperative systemic chemotherapy alters the histopathological growth patterns of colorectal liver metastases. J Pathol Clin Res. 2022;8:48-64. [PubMed] [DOI] |
| 39. | Meyer Y, Bohlok A, Höppener D, Galjart B, Doukas M, Grünhagen DJ, Labar A, Lucidi V, Vermeulen PB, Verhoef C, Donckier V. Histopathological growth patterns of resected non-colorectal, non-neuroendocrine liver metastases: a retrospective multicenter study. Clin Exp Metastasis. 2022;39:433-442. [PubMed] [DOI] |
| 40. | Li Z, Nguyen Canh H, Takahashi K, Le Thanh D, Nguyen Thi Q, Yang R, Yoshimura K, Sato Y, Nguyen Thi K, Nakata H, Ikeda H, Kozaka K, Kobayashi S, Yagi S, Harada K. Histopathological growth pattern and vessel co-option in intrahepatic cholangiocarcinoma. Med Mol Morphol. 2024;57:200-217. [PubMed] [DOI] |
| 41. | Qi M, Fan S, Huang M, Pan J, Li Y, Miao Q, Lyu W, Li X, Deng L, Qiu S, Liu T, Deng W, Chu X, Jiang C, He W, Xia L, Yang Y, Hong J, Qi Q, Yin W, Liu X, Shi C, Chen M, Ye W, Zhang D. Targeting FAPα-expressing hepatic stellate cells overcomes resistance to antiangiogenics in colorectal cancer liver metastasis models. J Clin Invest. 2022;132. [PubMed] [DOI] |
| 42. | Kuczynski EA, Vermeulen PB, Pezzella F, Kerbel RS, Reynolds AR. Vessel co-option in cancer. Nat Rev Clin Oncol. 2019;16:469-493. [PubMed] [DOI] |
| 43. | Lugassy C, Vermeulen PB, Ribatti D, Pezzella F, Barnhill RL. Vessel co-option and angiotropic extravascular migratory metastasis: a continuum of tumour growth and spread? Br J Cancer. 2022;126:973-980. [PubMed] [DOI] |
| 44. | Kuczynski EA, Yin M, Bar-Zion A, Lee CR, Butz H, Man S, Daley F, Vermeulen PB, Yousef GM, Foster FS, Reynolds AR, Kerbel RS. Co-option of Liver Vessels and Not Sprouting Angiogenesis Drives Acquired Sorafenib Resistance in Hepatocellular Carcinoma. J Natl Cancer Inst. 2016;108. [PubMed] [DOI] |
| 45. | Kuczynski EA, Kerbel RS. Implications of vessel co-option in sorafenib-resistant hepatocellular carcinoma. Chin J Cancer. 2016;35:97. [PubMed] [DOI] |
| 46. | Zhang N, Zhang S, Wu W, Lu W, Jiang M, Zheng N, Huang J, Wang L, Liu H, Zheng M, Wang J. Regorafenib inhibits migration, invasion, and vasculogenic mimicry of hepatocellular carcinoma via targeting ID1-mediated EMT. Mol Carcinog. 2021;60:151-163. [PubMed] [DOI] |
| 47. | Huang X, Xiang L, Wang B, Hu J, Liu C, Ren A, Du K, Ye G, Liang Y, Tang Y, Yang D, Yuan Y. CMTM6 promotes migration, invasion, and EMT by interacting with and stabilizing vimentin in hepatocellular carcinoma cells. J Transl Med. 2021;19:120. [PubMed] [DOI] |
| 48. | Aiello NM, Maddipati R, Norgard RJ, Balli D, Li J, Yuan S, Yamazoe T, Black T, Sahmoud A, Furth EE, Bar-Sagi D, Stanger BZ. EMT Subtype Influences Epithelial Plasticity and Mode of Cell Migration. Dev Cell. 2018;45:681-695.e4. [PubMed] [DOI] |
| 49. | Rada M, Lazaris A, Kapelanski-Lamoureux A, Mayer TZ, Metrakos P. Tumor microenvironment conditions that favor vessel co-option in colorectal cancer liver metastases: A theoretical model. Semin Cancer Biol. 2021;71:52-64. [PubMed] [DOI] |
| 50. | He B, Ganss R. Modulation of the Vascular-Immune Environment in Metastatic Cancer. Cancers (Basel). 2021;13. [PubMed] [DOI] |
| 51. | Angelova M, Mlecnik B, Vasaturo A, Bindea G, Fredriksen T, Lafontaine L, Buttard B, Morgand E, Bruni D, Jouret-Mourin A, Hubert C, Kartheuser A, Humblet Y, Ceccarelli M, Syed N, Marincola FM, Bedognetti D, Van den Eynde M, Galon J. Evolution of Metastases in Space and Time under Immune Selection. Cell. 2018;175:751-765.e16. [PubMed] [DOI] |
| 52. | Rahma OE, Hodi FS. The Intersection between Tumor Angiogenesis and Immune Suppression. Clin Cancer Res. 2019;25:5449-5457. [PubMed] [DOI] |
| 53. | Latacz E, Caspani E, Barnhill R, Lugassy C, Verhoef C, Grünhagen D, Van Laere S, Fernández Moro C, Gerling M, Dirix M, Dirix LY, Vermeulen PB. Pathological features of vessel co-option versus sprouting angiogenesis. Angiogenesis. 2020;23:43-54. [PubMed] [DOI] |
| 54. | Ribatti D, Pezzella F. Vascular Co-Option and Other Alternative Modalities of Growth of Tumor Vasculature in Glioblastoma. Front Oncol. 2022;12:874554. [PubMed] [DOI] |
| 55. | Fukumura D, Kloepper J, Amoozgar Z, Duda DG, Jain RK. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol. 2018;15:325-340. [PubMed] [DOI] |
| 56. | Voron T, Colussi O, Marcheteau E, Pernot S, Nizard M, Pointet AL, Latreche S, Bergaya S, Benhamouda N, Tanchot C, Stockmann C, Combe P, Berger A, Zinzindohoue F, Yagita H, Tartour E, Taieb J, Terme M. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med. 2015;212:139-148. [PubMed] [DOI] |
| 57. | Zeng H, Hou Y, Zhou X, Lang L, Luo H, Sun Y, Wan X, Yuan T, Wang R, Liu Y, Tang R, Cheng S, Xu M, Liu M. Cancer-associated fibroblasts facilitate premetastatic niche formation through lncRNA SNHG5-mediated angiogenesis and vascular permeability in breast cancer. Theranostics. 2022;12:7351-7370. [PubMed] [DOI] |
| 58. | Dou R, Liu K, Yang C, Zheng J, Shi D, Lin X, Wei C, Zhang C, Fang Y, Huang S, Song J, Wang S, Xiong B. EMT-cancer cells-derived exosomal miR-27b-3p promotes circulating tumour cells-mediated metastasis by modulating vascular permeability in colorectal cancer. Clin Transl Med. 2021;11:e595. [PubMed] [DOI] |
| 59. | Hato T, Zhu AX, Duda DG. Rationally combining anti-VEGF therapy with checkpoint inhibitors in hepatocellular carcinoma. Immunotherapy. 2016;8:299-313. [PubMed] [DOI] |
| 60. | EGFR Inhibitors Synergize with Lenvatinib for Liver Cancer Treatment. Cancer Discov. 2021;11:2124. [PubMed] [DOI] |
| 61. | Anwanwan D, Singh SK, Singh S, Saikam V, Singh R. Challenges in liver cancer and possible treatment approaches. Biochim Biophys Acta Rev Cancer. 2020;1873:188314. [PubMed] [DOI] |
| 62. | Shang R, Song X, Wang P, Zhou Y, Lu X, Wang J, Xu M, Chen X, Utpatel K, Che L, Liang B, Cigliano A, Evert M, Calvisi DF, Chen X. Cabozantinib-based combination therapy for the treatment of hepatocellular carcinoma. Gut. 2021;70:1746-1757. [PubMed] [DOI] |
| 63. | Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168:707-723. [PubMed] [DOI] |
| 64. | Lu C, Rong D, Zhang B, Zheng W, Wang X, Chen Z, Tang W. Current perspectives on the immunosuppressive tumor microenvironment in hepatocellular carcinoma: challenges and opportunities. Mol Cancer. 2019;18:130. [PubMed] [DOI] |
| 65. | Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, Coussens LM, Gabrilovich DI, Ostrand-Rosenberg S, Hedrick CC, Vonderheide RH, Pittet MJ, Jain RK, Zou W, Howcroft TK, Woodhouse EC, Weinberg RA, Krummel MF. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24:541-550. [PubMed] [DOI] |
| 66. | Leone RD, Powell JD. Metabolism of immune cells in cancer. Nat Rev Cancer. 2020;20:516-531. [PubMed] [DOI] |
| 67. | Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res. 2010;86:226-235. [PubMed] [DOI] |
| 68. | Ribatti D, Pezzella F. Overview on the Different Patterns of Tumor Vascularization. Cells. 2021;10. [PubMed] [DOI] |
| 69. | Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197-218. [PubMed] [DOI] |
| 70. | Annese T, Errede M, d'Amati A, De Giorgis M, Lorusso L, Tamma R, Ribatti D. Differential P-Glycoprotein/CD31 Expression as Markers of Vascular Co-Option in Primary Central Nervous System Tumors. Diagnostics (Basel). 2022;12. [PubMed] [DOI] |
| 71. | Yuan Y, He W, Yang Z, Qiu J, Huang Z, Shi Y, Lin Z, Zheng Y, Chen M, Lau WY, Li B, Yuan Y. TACE-HAIC combined with targeted therapy and immunotherapy versus TACE alone for hepatocellular carcinoma with portal vein tumour thrombus: a propensity score matching study. Int J Surg. 2023;109:1222-1230. [PubMed] [DOI] |
| 72. | Yang Y, Xiong L, Li M, Jiang P, Wang J, Li C. Advances in radiotherapy and immunity in hepatocellular carcinoma. J Transl Med. 2023;21:526. [PubMed] [DOI] |
| 73. | Elms D, Badami A, Dhanarajan A. Systemic Therapy in Metastatic Hepatocellular Carcinoma. Curr Gastroenterol Rep. 2022;24:65-71. [PubMed] [DOI] |
| 74. | 倪 爽, 吕 家华. 饮食调节与肿瘤放射治疗. 肿瘤代谢与营养电子杂志. 2024;11:28-34. [DOI] |
| 75. | Lu J, Ye X, Fan F, Xia L, Bhattacharya R, Bellister S, Tozzi F, Sceusi E, Zhou Y, Tachibana I, Maru DM, Hawke DH, Rak J, Mani SA, Zweidler-McKay P, Ellis LM. Endothelial cells promote the colorectal cancer stem cell phenotype through a soluble form of Jagged-1. Cancer Cell. 2013;23:171-185. [PubMed] [DOI] |
| 76. | Lorente S, Hautefeuille M, Sanchez-Cedillo A. The liver, a functionalized vascular structure. Sci Rep. 2020;10:16194. [PubMed] [DOI] |
| 77. | Jeong HS, Jones D, Liao S, Wattson DA, Cui CH, Duda DG, Willett CG, Jain RK, Padera TP. Investigation of the Lack of Angiogenesis in the Formation of Lymph Node Metastases. J Natl Cancer Inst. 2015;107. [PubMed] [DOI] |
| 78. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [PubMed] [DOI] |
| 79. | Majidpoor J, Mortezaee K. Angiogenesis as a hallmark of solid tumors - clinical perspectives. Cell Oncol (Dordr). 2021;44:715-737. [PubMed] [DOI] |
| 80. | Berghoff AS, Rajky O, Winkler F, Bartsch R, Furtner J, Hainfellner JA, Goodman SL, Weller M, Schittenhelm J, Preusser M. Invasion patterns in brain metastases of solid cancers. Neuro Oncol. 2013;15:1664-1672. [PubMed] [DOI] |
| 81. | Eefsen RL, Vermeulen PB, Christensen IJ, Laerum OD, Mogensen MB, Rolff HC, Van den Eynden GG, Høyer-Hansen G, Osterlind K, Vainer B, Illemann M. Growth pattern of colorectal liver metastasis as a marker of recurrence risk. Clin Exp Metastasis. 2015;32:369-381. [PubMed] [DOI] |
| 82. | Abe H, Yasunaga Y, Yamazawa S, Nakai Y, Gonoi W, Nishioka Y, Murono K, Sasaki K, Arita J, Kawai K, Nozawa H, Hasegawa K, Ishihara S, Ushiku T. Histological growth patterns of colorectal cancer liver metastases: a strong prognostic marker associated with invasive patterns of the primary tumor and p53 alteration. Hum Pathol. 2022;123:74-83. [PubMed] [DOI] |
| 83. | Er EE, Valiente M, Ganesh K, Zou Y, Agrawal S, Hu J, Griscom B, Rosenblum M, Boire A, Brogi E, Giancotti FG, Schachner M, Malladi S, Massagué J. Pericyte-like spreading by disseminated cancer cells activates YAP and MRTF for metastatic colonization. Nat Cell Biol. 2018;20:966-978. [PubMed] [DOI] |
| 84. | Carbonell WS, DeLay M, Jahangiri A, Park CC, Aghi MK. β1 integrin targeting potentiates antiangiogenic therapy and inhibits the growth of bevacizumab-resistant glioblastoma. Cancer Res. 2013;73:3145-3154. [PubMed] [DOI] |
学科分类: 胃肠病学和肝病学
手稿来源地: 黑龙江省
同行评议报告学术质量分类
A级 (优秀): 0
B级 (非常好): B, B, B
C级 (良好): C
D级 (一般): 0
E级 (差): 0
科学编辑: 张砚梁 制作编辑:张砚梁