修回日期: 2007-10-04
接受日期: 2007-10-28
在线出版日期: 2007-10-18
研究不同浓度曲格列酮对胃癌MKN45细胞过氧化物酶体增殖激活受体γ(PPARγ)激活水平变化及细胞凋亡的作用.
方法: 胃癌MKN45细胞培养后分为4组, 分别为生理盐水对照组和1、5、10 μmol/L曲格列酮实验组. 用EMSA测定不同浓度曲格列酮对胃癌MKN45细胞PPARγ激活水平的变化, 用流式细胞术检测caspase-3蛋白的表达、细胞周期的变化及凋亡.
结果: 随曲格列酮浓度的增加, PPARγ活性明显升高, 以对照组活性为100, 1、5、10 μmol/L曲格列酮组的PPARγ平均活性分别为155.8、218.7和307.6(P<0.01). 流式细胞术证实MKN45细胞经1, 5, 10 μmol/L曲格列酮的作用后, 随着曲格列酮剂量的增加, Caspase-3蛋白的表达均值分别为4.51, 10.95, 20.49, 33.56(P<0.01), G0/G1期细胞逐渐增加, S期细胞下降, 凋亡细胞增多.
结论: 曲格列酮通过对胃癌MKN45细胞PPARγ的激活可引发胃癌MKN45细胞株细胞周期抑制及凋亡, 可为胃癌的治疗提供新的靶点.
引文著录: 蒋春舫, 陈庆, 周洁, 郑海, 陈娟. 曲格列酮诱导胃癌MKN45细胞株凋亡. 世界华人消化杂志 2007; 15(29): 3074-3078
Revised: October 4, 2007
Accepted: October 28, 2007
Published online: October 18, 2007
AIM: To observe the effects of different concentrations of troglitazone on the activity of peroxisome proliferators-activated receptor γ (PPARγ), and apoptosis of the MKN45 gastric cell line.
METHODS: Cultured MKN45 gastric cells were randomly divided into four groups: control, 1, 5 and 10 μmol/L troglitazone. The change in activity of PPARγ was detected by electrophoretic mobility shift assay (EMSA). Flow cytometry was used to detect changes in the expression of caspase-3, cell cycle and apoptosis.
RESULTS: TPPARγ activity of cultured MKN45 gastric cells increased significantly with the concentration of troglitazone. Data were quantified for the control group as 100, the mean activity of 1 μmol/L troglitazone was 155.8, 5 μmol/L was 218.7, and 10 μmol/L was 307.6 (P < 0.01). Flow cytometry confirmed that the mean expression of caspase-3 protein was increased to 4.51, 10.95, 20.49, 33.56 with increasing concentration of troglitazone. The cells in G0/G1 stage increased steadily and those in S stage decreased at the same time (P < 0.01). The rate of apoptosis also increased.
CONCLUSION: Troglitazone could activate PPARγ and cell-cycle arrest and apoptosis of MKN45 gastric cells. PPARγ may become a new target in the treatment of gastric cancer.
- Citation: Jiang CF, Chen Q, Zhou J, Zheng H, Chen J. Troglitazone induces gastric cancer cell line MKN45 apoptosis. Shijie Huaren Xiaohua Zazhi 2007; 15(29): 3074-3078
- URL: https://www.wjgnet.com/1009-3079/full/v15/i29/3074.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i29.3074
胃癌是我国最常见的消化系肿瘤, 其发病机制尚未阐明. 胃癌在早期即可发生转移, 对放疗敏感性差, 生存率低. 研究证实, 过氧化物酶体增殖激活受体γ(peroxisome proliferation activated receptor γ, PPARγ)与多种癌症的发生、发展密切相关[1-4]. PPAR属于激素核受体超家族, 能被某些降脂药物、苯氧乙酸脂和脂肪酸等物质激活[5-8]. PPAR基因在不同物种中存在几种亚型, 包括较为清楚的α, β, γ 3种亚型和δ亚型[9]. 最新研究初步证实, PPARγ的激活可促进大肠癌、乳腺癌等肿瘤细胞系的分化, 抑制肿瘤细胞系的增殖, 并可导致肿瘤细胞系凋亡[10-11]. 我们研究曲格列酮(troglitazone)对胃癌MKN45细胞PPARγ激活水平变化及细胞凋亡的作用, 为临床胃癌的辅助治疗提供新的靶点.
PPARγ寡聚核苷酸探针(Invitrogen CO.), Caspase-3 antibody(Santa Cruz), 32P-ATP(中科院高能物理所), T4-多聚核苷酸激酶(Promega), 二硫苏糖醇(DTT)和苯甲基磺酰氟(PMSF)(Sigma); NP-40(Fluka), Sephadex G-50(Pharmacia), X光底片(Kodak), HEPES, RPMI 1640培养基(中健公司), 显影及定影试剂(乐凯), 胃癌MKN45细胞株(同济医学院生化教研室惠赠).
胃癌MKN45细胞株常规RPMI 1640培养基加100 mL/L胎牛血清培养, 70%传代, 大量扩增, 随机分4组: 即生理盐水对照组和1, 5, 10 μmol/L曲格列酮实验组, 分别给药. 经过处理24 h的细胞株经胰酶消化, 洗涤(TBS), 离心(1000 r/min×5 min), 收集处理后的细胞, TBS 10 mL重悬并计数, 至少达5×108 /L, 再次离心后弃上清, 加入TBS 1 mL重悬沉淀并转移至1.5 mL EP管中, 置4℃使之温度平衡. 4℃, 3000 r/min离心15 s, 小心弃上清; 用冰冷的Buffer A 400 μL小心重悬沉淀, 置冰浴15 min使细胞肿胀; 加入NP-40使其终浓度达1 g/L, 剧烈振荡裂解细胞; 4℃, 3000 r/min离心4 min, 小心弃净上清. 用冰冷的Buffer C 50 μL小心重悬沉淀, 4℃剧烈振摇15 min; 4℃, 12 000 r/min离心10 min; 上清经考马斯亮兰G-250法测定蛋白质浓度后, 各取核抽提物8 μg, 与32P-ATP标记的PPARγ寡聚核苷酸探针1 μg结合, 4℃下行EMSA, 2 h后凝胶取出, 置干胶仪干胶, -70℃放射自显影[12]. 另各组细胞培养48 h后收集, 40 g/L多聚甲醛室温下固定30 min, 再用PBS洗3次, 每次5 min, 加入抗caspase-3的一抗, 置室温60 min, PBS洗3遍, 加入FITC标记的二抗, 室温避光反应30 min. PBS洗3次后, 移入测试管, 上机检测细胞平均荧光强度. 阴性对照不加FITC标记的二抗, 代之以同型IgG. 避光30 min后用流式细胞仪检测. 每管计数10 000个细胞. 离心收集细胞, 调整浓度为1×109 /L, -20℃的700 mL/L乙醇固定1 h; -20℃冰箱保存待染色. 0.01 mol/L PBS(pH7.2)洗涤2次, 离心弃上清, 加入PBS 1 mL溶解细胞沉淀; 加入RNase至终浓度100 mg/L, 37℃ 孵育30 min; 然后加入PI染液至终浓度5 mg/L混匀, 4℃避光染色30 min. 取经PI新近染色的胃癌细胞悬液1 mL(细胞数10 000个左右), 在FCM下检测. PI及annexin V抗体双染胃癌细胞, 流式细胞术检测凋亡[13].
统计学处理 影像使用Scion Image软件量化, 利用SPSS10.0软件包进行统计学处理, 数据采用各组的mean±SD表示, 各组间比较采用t检验.
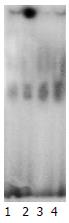
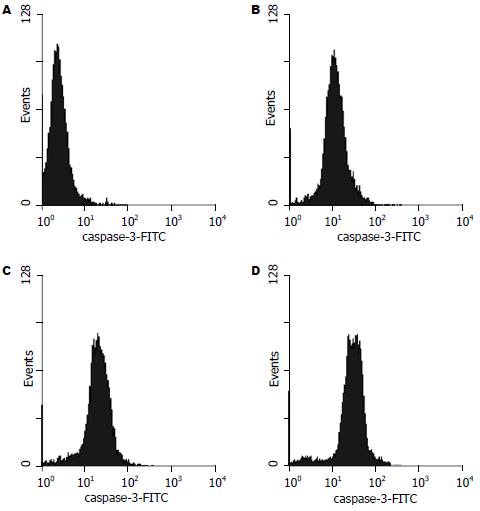
随曲格列酮浓度的增加, PPARγ活性明显升高, 以生理盐水对照组活性均数为100, 1, 5, 10 μmol/L曲格列酮组PPARγ活性均数分别为155.8, 218.7和307.6(P<0.01, 图1). 流式细胞术证实MKN45细胞经1, 5, 10 μmol/L曲格列酮的作用后, 随着曲格列酮剂量的增加, caspase-3蛋白的表达均值逐渐增加, 分别为4.51, 10.95, 20.49和33.56, 各组间存在显著性差异(P<0.01, 图2).
PPAR基因在不同物种中存在α, β, γ和δ 4种亚型, 同其他核受体超家族成员一样, PPAR有6个调节功能区: 即A, B, C, D, E, F[9,14]. 过氧化物酶体增殖激活受体γ是配体活化的核受体, 配体与之结合后, 活化PPARγ并与PPAR反应元件(peroxisome proliferator-activated receptor response element, PPRE)结合, 调节基因转录. PPAR通过PPAR-RXR(retinoid-X receptor)二聚体与一个相应元件结合而调控基因的转录, 该元件是由间隔一个核苷酸的PuGGTCA的重复序列组成, 该受体因这些物质也能够促使过氧化物酶体(peroxisome)体积和含量增加而得名. 核激素受体至少有两个二聚作用界面: 一个在他的DNA结合域, 另一个在他的配体结合域. DNA结合域在没有DNA存在时是单体, 有DNA时才发生聚合. 配体结合域二聚作用的功能看来是稳定受体-DNA复合物. PPAR能够调控许多参与细胞内外代谢的目的基因, 尤其是β-氧化过程中的一些重要酶类, 另外PPAR也能够参与脂肪细胞的分化[15-17]. 最新研究初步证实, PPARγ的激活可促进包括大肠癌、乳腺癌等肿瘤细胞系的分化, 抑制肿瘤细胞系的增殖, 并可导致肿瘤细胞系凋亡[18-22].
曲格列酮是PPARγ的配体, 其格列酮(glitazones)部分能与PPAR受体相结合. PPAR受体通过打开和关闭特定的基因来调控基因表达[23-25]. Glitazones含有一个噻唑烷二酮(thiazolidinedione)环的化学集团, 能够和PPAR紧密结合并激活PPAR[26-30]. 我们证实, 曲格列酮浓度的增加可明显升高PPARγ活性, 以对照组活性均值为100, 1 μmol/L曲格列酮组PPARγ活性均值为155.8, 5 μmol/L曲格列酮组PPARγ活性均值为218.7, 10 μmol/L曲格列酮组PPARγ活性均值为307.6(P<0.01). 流式细胞术证实MKN45细胞经1, 5, 10 μmol/L曲格列酮的作用时, 随着曲格列酮剂量的增加, G0/G1期细胞逐渐增加, S期细胞下降, Caspase-3蛋白的表达均值分别为4.51, 10.95, 20.49和33.56(P<0.01). 另外, MKN45细胞经1, 5, 10 μmol/L曲格列酮的作用后, 随着曲格列酮剂量的增加, 凋亡细胞明显增多, 凋亡率分别为1.07%, 11.63%, 12.96%和23.29%(P<0.01).
本实验显示, PPARγ激活能够导致肿瘤细胞周期抑制, 并诱导肿瘤细胞凋亡. PPARγ激活的体外试验结果证实其具有抑瘤功能, 给进一步的研究提供了基础和方向. PPARγ激活调控参与许多细胞内外代谢的目的基因, 最终导致肿瘤细胞周期抑制; 诱导肿瘤细胞凋亡的准确途径是进一步的研究目标, 最终可能为临床防治胃癌提供新的药物及靶点.
胃癌是我国最常见的消化系肿瘤, 其发病机制尚未完全阐明, 早期预防及治疗极其重要. 最新研究证实, 过氧化物酶体增殖激活受体PPARγ与多种癌症的发生、发展密切相关.
过氧化物酶体增殖激活受体具有6个调节功能区, 即A、B、C、D、E、F, 其中C和E是DNA和配体结合区. 过氧化物酶体增殖激活受体γ是配体活化的核受体, 配体与之结合后, 活化PPARγ并与PPAR反应元件结合, 调节基因转录. 最新研究初步证实, PPAR-γ的激活可促进结肠癌、乳腺癌等肿瘤细胞系的分化, 抑制肿瘤细胞系的增殖,并可导致肿瘤细胞系凋亡.
本文研究了曲格列酮对胃癌细胞MKN45的凋亡诱导作用, 目前此类研究较少, 内容新颖, 行文流畅, 结构层次较清晰, 有一定的参考价值.
| 1. | Sporn MB, Suh N, Mangelsdorf DJ. Prospects for prevention and treatment of cancer with selective PPARgamma modulators (SPARMs). Trends Mol Med. 2001;7:395-400. [PubMed] |
| 2. | Bonofiglio D, Gabriele S, Aquila S, Catalano S, Gentile M, Middea E, Giordano F, Ando S. Estrogen receptor alpha binds to peroxisome proliferator-activated receptor response element and negatively interferes with peroxisome proliferator-activated receptor gamma signaling in breast cancer cells. Clin Cancer Res. 2005;11:6139-6047. [PubMed] |
| 3. | Loy CJ, Evelyn S, Lim FK, Liu MH, Yong EL. Growth dynamics of human leiomyoma cells and inhibitory effects of the peroxisome proliferator-activated receptor-gamma ligand, pioglitazone. Mol Hum Reprod. 2005;11:561-566. [PubMed] |
| 4. | Fenner MH, Elstner E. Peroxisome proliferator-activated receptor-gamma ligands for the treatment of breast cancer. Expert Opin Investig Drugs. 2005;14:557-568. [PubMed] |
| 5. | Planaguma A, Claria J, Miquel R, Lopez-Parra M, Titos E, Masferrer JL, Arroyo V, Rodes J. The selective cyclooxygenase-2 inhibitor SC-236 reduces liver fibrosis by mechanisms involving non-parenchymal cell apoptosis and PPARgamma activation. FASEB J. 2005;19:1120-1122. [PubMed] |
| 6. | Knight B, Yeap BB, Yeoh GC, Olynyk JK. Inhibition of adult liver progenitor (oval) cell growth and viability by an agonist of the peroxisome proliferator activated receptor (PPAR) family member gamma, but not alpha or delta. Carcinogenesis. 2005;26:1782-1792. [PubMed] |
| 7. | Luquet S, Gaudel C, Holst D, Lopez-Soriano J, Jehl-Pietri C, Fredenrich A, Grimaldi PA. Roles of PPAR delta in lipid absorption and metabolism: a new target for the treatment of type 2 diabetes. Biochim Biophys Acta. 2005;1740:313-317. [PubMed] |
| 8. | Farmer SR. Regulation of PPARgamma activity during adipogenesis. Int J Obes (Lond). 2005;29 Suppl 1:S13-16. [PubMed] |
| 9. | Palakurthi SS, Aktas H, Grubissich LM, Mortensen RM, Halperin JA. Anticancer effects of thiazolidinediones are independent of peroxisome proliferator-activated receptor gamma and mediated by inhibition of translation initiation. Cancer Res. 2001;61:6213-6218. [PubMed] |
| 10. | Osawa E, Nakajima A, Wada K, Ishimine S, Fujisawa N, Kawamori T, Matsuhashi N, Kadowaki T, Ochiai M, Sekihara H. Peroxisome proliferator-activated receptor gamma ligands suppress colon carcinogenesis induced by azoxymethane in mice. Gastroenterology. 2003;124:361-367. [PubMed] |
| 11. | Suh N, Wang Y, Williams CR, Risingsong R, Gilmer T, Willson TM, Sporn MB. A new ligand for the peroxisome proliferator-activated receptor-gamma (PPAR-gamma), GW7845, inhibits rat mammary carcinogenesis. Cancer Res. 1999;59:5671-5673. [PubMed] |
| 12. | Shimada T, Fujii Y, Koike T, Tabei K, Namatame T, Yamagata M, Tajima A, Yoneda M, Terano A, Hiraishi H. Peroxisome proliferator-activated receptor gamma (PPARgamma) regulates trefoil factor family 2 (TFF2) expression in gastric epithelial cells. Int J Biochem Cell Biol. 2007;39:626-637. [PubMed] |
| 13. | Gupta S, Ahmad N, Nieminen AL, Mukhtar H. Growth inhibition, cell-cycle dysregulation, and induction of apoptosis by green tea constituent (-)-epigallocatechin-3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol Appl Pharmacol. 2000;164:82-90. [PubMed] |
| 14. | Miyachi H, Uchiki H. Analysis of the critical structural determinant(s) of species-selective peroxisome proliferator-activated receptor alpha (PPAR alpha)-activation by phenylpropanoic acid-type PPAR alpha agonists. Bioorg Med Chem Lett. 2003;13:3145-3149. [PubMed] |
| 15. | He W, Barak Y, Hevener A, Olson P, Liao D, Le J, Nelson M, Ong E, Olefsky JM, Evans RM. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci U S A. 2003;100:15712-15717. [PubMed] |
| 16. | Dolinsky VW, Gilham D, Hatch GM, Agellon LB, Lehner R, Vance DE. Regulation of triacylglycerol hydrolase expression by dietary fatty acids and peroxisomal proliferator-activated receptors. Biochim Biophys Acta. 2003;1635:20-28. [PubMed] |
| 17. | Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol. 2004;24:3057-3067. [PubMed] |
| 18. | Yao CJ, Lai GM, Chan CF, Cheng AL, Yang YY, Chuang SE. Dramatic synergistic anticancer effect of clinically achievable doses of lovastatin and troglitazone. Int J Cancer. 2006;118:773-779. [PubMed] |
| 19. | Ray DM, Akbiyik F, Phipps RP. The peroxisome proliferator-activated receptor gamma (PPARgamma) ligands 15-deoxy-Delta12,14-prostaglandin J2 and ciglitazone induce human B lymphocyte and B cell lymphoma apoptosis by PPARgamma-independent mechanisms. J Immunol. 2006;177:5068-5076. [PubMed] |
| 20. | Kim HJ, Woo IS, Kang ES, Eun SY, Kim GH, Ham SA, Kim HJ, Lee JH, Chang KC, Kim JH. Phorbol ester potentiates the growth inhibitory effects of troglitazone via up-regulation of PPARgamma in A549 cells. Biochem Biophys Res Commun. 2006;349:660-667. [PubMed] |
| 21. | Ware JH, Zhou Z, Kopelovich L, Kennedy AR. Evaluation of cancer chemopreventive agents using clones derived from a human prostate cancer cell line. Anticancer Res. 2006;26:4177-4183. [PubMed] |
| 22. | Li M, Lee TW, Yim AP, Mok TS, Chen GG. Apoptosis induced by troglitazone is both peroxisome proliferator-activated receptor-gamma- and ERK-dependent in human non-small lung cancer cells. J Cell Physiol. 2006;209:428-438. [PubMed] |
| 23. | Desmet C, Warzee B, Gosset P, Melotte D, Rongvaux A, Gillet L, Fievez L, Seumois G, Vanderplasschen A, Staels B. Pro-inflammatory properties for thiazolidinediones. Biochem Pharmacol. 2005;69:255-265. [PubMed] |
| 24. | Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005;130:685-696. [PubMed] |
| 25. | Kobuke T, Tazuma S, Hyogo H, Chayama K. A Ligand for peroxisome proliferator-activated receptor gamma inhibits human cholan-giocarcinoma cell growth: potential molecular targeting strategy for cholangioma. Dig Dis Sci. 2006;51:1650-1657. [PubMed] |
| 27. | Irons BK, Mazzolini TA, Greene RS. Delaying the onset of type 2 diabetes mellitus in patients with prediabetes. Pharmacotherapy. 2004;24:362-371. [PubMed] |
| 28. | Boileau C, Martel-Pelletier J, Fahmi H, Mineau F, Boily M, Pelletier JP. The peroxisome proliferator-activated receptor gamma agonist pioglitazone reduces the development of cartilage lesions in an experimental dog model of osteoarthritis: in vivo protective effects mediated through the inhibition of key signaling and catabolic pathways. Arthritis Rheum. 2007;56:2288-2298. [PubMed] |
| 29. | Boyd AS. Thiazolidinediones in dermatology. Int J Dermatol. 2007;46:557-563. [PubMed] |
| 30. | Sarafidis PA, Bakris GL. Protection of the kidney by thiazolidinediones: an assessment from bench to bedside. Kidney Int. 2006;70:1223-1233. [PubMed] |
编辑: 何燕 电编:郭海丽