修回日期: 2005-11-17
接受日期: 2005-12-02
在线出版日期: 2006-02-18
目的: 研究中药复方肝癌-1号对Wistar大鼠巨噬细胞杀伤肝癌细胞HepG2能力的影响并探讨其可能机制.
方法: 用0, 25, 50, 75, 100 mmol/L肝癌-1号提纯液处理Wistar大鼠巨噬细胞24 h后, 用四甲基偶氮唑盐法(MTT), 检测不同浓度药物处理后的巨噬细胞对HepG2细胞的杀伤能力; RT-PCR法检测各组巨噬细胞TNF-α, NF-κB和iNOS mRNA表达.
结果: 肝癌-1号处理后, 巨噬细胞对肿瘤抑制率随药物剂量的升高而升高, 与0 mmol/L组相比, 100, 75 mmol/L浓度时, 巨噬细胞对HepG2细胞具有明显的杀伤作用, 肿瘤细胞抑制率分别为28.0%±4.5%、23.5%±3.4%(P<0.05); 同时TNF-α, NF-κB和iNOS mRNA表达增强.
结论: 肝癌-1号通过诱导巨噬细胞TNF-α, NF-κB和iNOS mRNA的表达, 增强巨噬细胞对肝癌细胞HepG2的杀伤能力, 其抗癌效应可能与其参与机体免疫调节作用有关.
引文著录: 毕明星, 刘作金, 王华丽, 时毓君, 龚建平. 肝癌-1号对大鼠巨噬细胞杀伤肝癌细胞能力的影响及机制. 世界华人消化杂志 2006; 14(5): 526-529
Revised: November 17, 2005
Accepted: December 2, 2005
Published online: February 18, 2006
AIM: To investigate the effect of GanAi-1 on the competence of macrophages' killing HepG2 cells in Wistar rats, and to explore its possible mechanism.
METHODS: After isolation from Wistar rats, the macrophages were treated with different concentrations of GanAi-1 (0, 25, 50, 75, 100 mmol/L) for 24 h, and the killing effect of the macrophages on HepG2 cells was evaluated by MTT assay. The mRNA expression of rat macrophage cytokine tumor necrosis factor-a (TNF-α), nuclear factor-kB (NF-κB) and inducible nitric oxide synthase (iNOS) was detected by reverse transcription polymerase chain reaction (RT-PCR).
RESULTS: The inhibitory rates of rat macrophages on the growth of HepG2 cells were increased with the elevated concentrations of GanAi-1 and the rates were 28.0% ± 4.5% and 23.5% ± 3.4%, respectively, when 100 and 75 mmol/LGanAi-1 were used, which were significantly higher than that in the empty control (P < 0.05). The mRNA expression of TNF-α, NF-κB and iNOS were also increased with the increase of GanAi-1 level.
CONCLUSION: GanAi-1 can promote the killing ability of rat macrophages to HepG2 cells by induction of TNF-α, NF-κBand iNOS expression.
- Citation: Bi MX, Liu ZJ, Wang HL, Shi YJ, Gong JP. Effect of GanAi-1 on competence of murine macrophage in killing hepatic carcinoma cells and its mechanism. Shijie Huaren Xiaohua Zazhi 2006; 14(5): 526-529
- URL: https://www.wjgnet.com/1009-3079/full/v14/i5/526.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v14.i5.526
目前, 中药仍是治疗中、晚期肝癌的重要辅助手段. 茯苓、白术、黄芪、茵陈是临床实践中最常用到的、并有确切抗肝癌疗效的中药组分[1-3]. 我们将上述4种药物制成"肝癌一号"复方制剂. 为给临床治疗提供理论依据, 我们观察了肝癌-1号胶囊对Wistar大鼠巨噬细胞杀伤HepG2肝癌细胞能力的影响, 并对相关机制进行了进一步的研究.
1.1.1 主要仪器和试剂: HepG2细胞株(由本实验室传代培养); Wistar大鼠(♂, 8-12 wk, 重庆医科大学实验动物中心); CO2培养箱(Forma, 3131, 美国); RPMI1640培养基(Gibco公司); 新生牛血清(华美公司); 肝癌-1号提取液(由本实验室提纯); RT-PCR试剂盒, Trizol RNA提取试剂盒(Gibco公司).
1.1.2 大鼠腹腔巨噬细胞收集、纯化和培养: Wistar鼠腹腔注射可溶性淀粉(45 g/L)2 mL/只, 3 d后收集腹腔液, 离心1 000 r/min×5 min, 以Hang's液洗3次, RPMI1640培养液调节细胞浓度为1×109个/L, 加入24孔培养板, 在37 ℃, 50 mL/L的CO2培养箱内培养2 h. 去除非黏附细胞, 所剩贴壁细胞, 经特异性酯酶染色, 证实95%以上为巨噬细胞.
1.2.1 肝癌-1号对Wistar大鼠巨噬细胞杀伤HepG2细胞能力的影响: 采用四甲基偶氮唑盐法(MTT). 稀释后的巨噬细胞, 加入24孔培养板, 每孔1 mL, 每组6个平行孔, 置37 ℃, 50 mL/L CO2温箱孵育2 h, 加入肝癌-1号提取液, 并调至终浓度分别为0, 25, 50, 75, 100 mmol/L, 继续孵育24 h,弃去培养液, PBS洗2遍, 加入1 mL浓度为2×108个/L的HepG2细胞, 再孵育24 h,于培养结束前4 h每孔加入20 mL MTT, 37 ℃温育, 吸出上清液, 加入200 mL二甲基亚砜, 溶解并摇匀, 用酶标仪在490 nm处测定每个小孔的吸光度(A)值, 肿瘤细胞抑制率 = 实验组细胞A值/对照组细胞A值×100%.
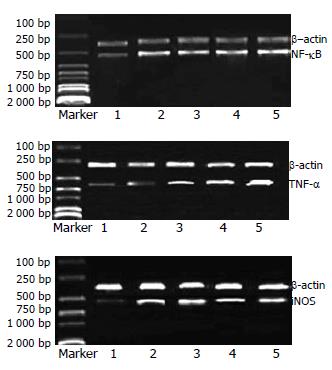
1.2.2 肝癌-1号对Wistar大鼠巨噬细胞NF-κB, TNF-α和iNOS mRNA表达的影响: 采用逆转录聚合酶链式反应(RT-PCR). 收集经不同浓度肝癌-1号提取液处理24 h的巨噬细胞, 参照Trizol试剂盒说明提取总RNA并逆转录为cDNA. 将逆转录产物行PCR: PCR反应体系20 mL, 1.5 mmol/L MgCl2, 200 mmol/L dNTP, 2.0 U Taq polymerase. PCR条件: 94 ℃变性5 min, 94 ℃ 30 s, 55 ℃ 30 s, 72 ℃ 60 s, 共30个循环. 最后72 ℃延伸10 min. 相应扩增引物序列如下: TNF-α上游引物: 5'-CCCTCACACTCAGATCATCTTCTCAA-3', 下游引物: 5'-TCTAAGTACTTGGGCAGGTTGACCTC-3'; NF-κB上游引物: 5'-ACGATCTGTTTCCCCTCATC-3', 下游引物: 5'-TGCTTCTCTCCCCAGCAATA-3'; iNOS上游引物5'-ACAACGTGGAGAAAACCCCAGGTG-3', 下游引物5'-ACAGCTCCGGGCATCGAAGACC-3' (由美国Gibco公司合成). 在检测各样本NF-κB, TNF-α和iNOS mRNA之前, 先采用b-action引物扩增各样本, 以对各样本的RNA进行计量标准化评估. PCR产物经20 g/L琼脂糖电泳后, 用凝胶分析系统进行分析.
统计学处理 采用SSPS 10.0软件分析数据, 结果用mean±SD表示, 采用t检验, P<0.05, 认为有显著性差异.
与肝癌-1号药物浓度为0%组相比,在100 mmol/L、75 mmol/ L浓度时, 巨噬细胞对HepG2细胞具有明显的杀伤作用, 肿瘤细胞抑制率分别为28%, 23.5%, (P<0.05); 而50 mmol/L和25 mmol/L的药物浓度不能使巨噬细胞显著杀伤HepG2细胞(P>0.10)(表1).
各实验浓度下, 均可见NF-κB, TNF-α和iNOS mRNA表达, 且随浓度增加, 表达呈增高趋势(图1).
传统中医认为肝癌为气滞血瘀、湿热聚毒、脾虚湿困、痰瘀毒阻结于胁下而成, 属本虚标实之证, 统计分析表明祛湿健脾理气为治疗肝癌的常用治法, 茯苓、白术、党参、黄芪等益气健脾祛湿中药为首选药物[1-3], 通过健脾理气、化淤散坚、清热解毒、扶正固本、祛湿等达到治疗目的. 作为中、晚期肝癌的辅助疗法, 上述药物的确有一定的临床疗效, 但具体作用的分子机制, 尚有待进一部的研究. 巨噬细胞是重要的免疫效应细胞, 研究表明许多抗癌中药是通过激活巨噬细胞, 增强巨噬细胞分泌细胞因子IL-1、IL-6、TNF-α, 释放NO等, 从而发挥其抗肿瘤的活性[4].
本实验中通过MTT法证实, 肝癌-1号也可活化巨噬细胞, 随药物浓度的增加, Wistar大鼠巨噬细胞杀伤HepG2细胞能力显著增强. 并且随药物浓度的增加, 巨噬细胞NF-κB, TNF-α和iNOS mRNA表达增多. NF-κB是普遍存在于细胞质中的一种快反应转录因子, 参与多种细胞因子基因的转录调控, 与免疫反应有密切关系. 静止状态下, 巨噬细胞内的NF-κB与抑制蛋白IkB结合, 以非活性的状态存在于大多数细胞的胞质中. TNF-α可激活NF-κB, 使IkB迅速磷酸化和降解, 被激活的NF-κB会进行核转移, 与DNA启动子上特定的认知序列结合, 转录靶基因, 诱导细胞产生更多的细胞因子如TNF-α[5,6], IL-2等及增强FC受体、Ia抗原表达, 显示出抗瘤活性. TNF-α对肿瘤细胞具有直接溶解及抗增殖作用, 进而引起肿瘤细胞坏死或导致肿瘤细胞凋亡[7-9]. 此外, TNF-α还具有免疫调节作用. 在TNF-α的作用下, 活化的巨噬细胞大量的表达iNOS并产生NO[10], 而NO是巨噬细胞杀灭肿瘤细胞的主要效应分子, 不仅可阻断肿瘤细胞的能量代谢和DNA复制, 抑制肿瘤细胞生长, 同时还诱导肿瘤细胞凋亡[11-15].
因此, 我们推测肝癌-1号可通过激活巨噬细胞, 增强其NF-κB, TNF-α及iNOS mRNA的表达, 进而直接杀灭或诱导肿瘤细胞凋亡, 来发挥其抗癌效应. 由于该组方为低毒的传统肝癌用药, 具有价廉、毒性低的优势, 作为无法手术切除的中, 晚期肝癌的辅助治疗手段, 显然具有进一步研究和推广的价值
茯苓、白术、黄芪、茵陈是临床实践中最常用到的、并有确切抗肝癌疗效的中药组分. 为给临床治疗提供理论依据, 实现中药现代化, 本文观察了上述4种成分的复方制剂对体外巨噬细胞杀伤HepG2肝癌细胞能力的影响.
从分子生物学角度, 观察了传统经验用中药对肿瘤杀伤细胞巨噬细胞活性的影响, 从而具体阐述了传统中药的抗癌机制.
本组方为低毒的传统肝癌用药, 具有价廉、毒性低的优势, 作为肝癌的辅助治疗手段, 具有广泛的研究和推广价值.
探讨中药的作用机理对于我国中药的发展非常重要. 本文对肝癌杀伤的作用机制进行了细胞学水平研究, 立题新颖, 具有一定的研究价值和指导意义.
| 4. | Shishodia S, Gutierrez AM, Lotan R, Aggarwal BB. N-(4-hydroxyphenyl)retinamide inhibits invasion, suppresses osteoclastogenesis, and potentiates apoptosis through down-regulation of I(kappa)B(alpha) kinase and nuclear factor-kappaB-regulated gene products. Cancer Res. 2005;65:9555-9565. [PubMed] |
| 5. | Ichikawa H, Takada Y, Murakami A, Aggarwal BB. Identification of a novel blocker of I kappa B alpha kinase that enhances cellular apoptosis and inhibits cellular invasion through suppression of NF-kappa B-regulated gene products. J Immunol. 2005;174:7383-7392. [PubMed] |
| 6. | Lee SB, Schorey JS. Activation and mitogen-activated protein kinase regulation of transcription factors Ets and NF-kappaB in Mycobacterium-infected macrophages and role of these factors in tumor necrosis factor alpha and nitric oxide synthase 2 promoter function. Infect Immun. 2005;73:6499-6507. [PubMed] |
| 7. | Campa VM, Iglesias JM, Carcedo MT, Rodriguez R, Riera J, Ramos S, Lazo PS. Polyinosinic acid induces TNF and NO production as well as NF-kappaB and AP-1 transcriptional activation in the monocytemacrophage cell line RAW 264.7. Inflamm Res. 2005;54:328-337. [PubMed] |
| 8. | Mishra S, Mishra JP, Gee K, McManus DC, LaCasse EC, Kumar A. Distinct role of calmodulin and calmodulin-dependent protein kinase-II in lipopolysaccharide and tumor necrosis factor-alpha-mediated suppression of apoptosis and antiapoptotic c-IAP2 gene expression in human monocytic cells. J Biol Chem. 2005;280:37536-37546. [PubMed] |
| 9. | Lyu SY, Park WB. Production of cytokine and NO by RAW 264.7 macrophages and PBMC in vitro incubation with flavonoids. Arch Pharm Res. 2005;28:573-581. [PubMed] |
| 10. | Hong SH, Seo SH, Lee JH, Choi BT. The aqueous extract from Artemisia capillaris Thunb. inhibits lipopolysaccharide-induced inflammatory response through preventing NF-kappaB activation in human hepatoma cell line and rat liver. Int J Mol Med. 2004;13:717-720. [PubMed] |
| 11. | Xidakis C, Kolios G, Valatas V, Notas G, Mouzas I, Kouroumalis E. Effect of octreotide on apoptosis-related proteins in rat Kupffer cells: a possible anti-tumour mechanism. Anticancer Res. 2004;24:833-841. [PubMed] |
| 12. | Chattopadhyay S, Das T, Sa G, Ray PK. Protein A-activated macrophages induce apoptosis in Ehrlich's ascites carcinoma through a nitric oxide-dependent pathway. Apoptosis. 2002;7:49-57. [PubMed] |
| 13. | Baratin M, Ziol M, Romieu R, Kayibanda M, Gouilleux F, Briand P, Leroy P, Haddada H, Renia L, Viguier M. Regression of primary hepatocarcinoma in cancer-prone transgenic mice by local interferon-gamma delivery is associated with macrophages recruitment and nitric oxide production. Cancer Gene Ther. 2001;8:193-202. [PubMed] |
| 14. | Nishikawa M, Sato EF, Kuroki T, Utsumi K, Inoue M. Macrophage-derived nitric oxide induces apoptosis of rat hepatoma cells in vivo. Hepatology. 1998;28:1474-1480. [PubMed] |
| 15. | Maekawa H, Iwabuchi K, Nagaoka I, Watanabe H, Kamano T, Tsurumaru M. Activated peritoneal macrophages inhibit the proliferation of rat ascites hepatoma AH-130 cells via the production of tumor necrosis factor-alpha and nitric oxide. Inflamm Res. 2000;49:541-547. [PubMed] |
电编: 张敏 编辑:菅鑫妍 审读:张海宁