修回日期: 2004-09-01
接受日期: 2004-09-24
在线出版日期: 2005-04-15
目的: 探讨细胞因子诱导的中性粒细胞趋化因子(CINC)和单核细胞趋化蛋白(MCP-1/JE)在大鼠急性坏死性胰腺炎(ANP)早期胰腺组织中的表达, 观察中药提取物氧化苦参碱(Oxy)对其表达水平的影响以及在ANP中的治疗作用.
方法: 以牛磺胆酸钠逆行胆胰管注射制备大鼠ANP模型, 56只SD大鼠随机分为假手术(SO)组, ANP 3 h, 6 h, 12 h组, Oxy治疗3 h, 6 h, 12 h组, 检测各组血清淀粉酶(Amy), 谷丙转氨酶( ALT)和肌酐(Cr)值, 观察胰腺组织病理学和胰腺组织中CINC及MCP-1/JE基因表达的改变.
结果: 比较SO组, CINC mRNA、MCP-1/JE mRNA在ANP各组胰腺组织中表达量显著增加(分别为0.61±0.08, 0.76±0.10, 0.89±0.12, 0.42±0.06, 0.65±0.08, 0.94±0.12, P<0.01), 随诱发ANP时间的进展有不断上调的趋势, 且都与胰腺组织病理学改变呈正相关(分别为0.89, 0.82, P<0.05), 与ANP各组比较, Oxy治疗各组CINC mRNA, MCP-1/JE mRNA表达量有明显下降(0.34±0.05, 0.45±0.06, 0.51±0.06, 0.31±0.04, 0.34±0.04, 0.36±0.05, P<0.01), 血清Amy, ALT和Cr值降低(分别为25.39±1.93 μkat, 37.66±4.27 μkat, 96.00±9.19 μkat, 94.5±58.3 nkat/L, 283.8±115.5 nkat/L, 469.7±157.3 nkat/L, 81.4±17.7 μmol/L, 89.1±18.7 μmol/L, 110.7±12.8 μmol/L, P<0.05或P<0.01), 胰腺组织病理学损害改善(5.95±0.17, 6.53±0.19, 7.58±0.20, P<0.01).
结论: CINC和MCP-1/JE是ANP早期重要的炎症反应递质, Oxy能抑制他们在ANP大鼠胰腺组织中的表达, 对ANP有一定的治疗作用.
引文著录: 李兆申, 许永春, 屠振兴, 施新岗. 急性坏死性胰腺炎早期胰腺组织趋化因子基因的表达及氧化苦参碱的影响. 世界华人消化杂志 2005; 13(8): 979-983
Revised: September 1, 2004
Accepted: September 24, 2004
Published online: April 15, 2005
AIM: To investigate the expression of cytokine-induced neutrophil chemoattractant (CINC) and monocyte chemoattractant protein-1/JE (MCP-1/JE) genes in the pancreas, and to assess the influence of Chinese herb extraction oxymatrine (Oxy) on the expression of CINC and MCP-1/JE genes and the therapeutic effects in early acute necrotizing pancreatitis (ANP).
METHODS: ANP was induced by retrograde infusion of 50 g/L sodium taurocholate into the biliary pancreatic duct of rats. Fifty-six rats were randomly divided into three groups: sham operation (SO) group, ANP 3 h, 6 h, 12 h groups, and Oxy 3 h, 6 h, 12 h groups. The activity of serum amylase (Amy), the serum levels of glutamic-pyruvic transaminase (ALT) and creatinine (Cr) were determined. Pathological changes of the pancreas were observed. The expression of CINC and MCP-1/JE genes in the pancreas was assayed.
RESULTS: Compared with that in SO group, the expression of CINC and MCP-1/JE genes in the pancreas in ANP groups were all increased greatly (0.61 ± 0.08, 0.76 ± 0.10, 0.89 ± 0.12, 0.42 ± 0.06, 0.65 ± 0.08, 0.94 ± 0.12, P < 0.01). Following the induction of ANP, the expression of CINC and MCP-1/JE genes was both up-regulated, which was significantly correlated with the pathological changes of the pancreas (r = 0.89 and 0.82, respectively, P < 0.05). Oxy attenuated the increase of CINC and MCP-1/JE gene expression (0.34 ± 0.05, 0.45 ± 0.06, 0.51 ± 0.06 vs 0.31 ± 0.04, 0.34 ± 0.04, 0.36 ± 0.05, in ANP 3 h, 6 h, 12 h groups and Oxy 3 h, 6 h, 12 h groups, respectively P < 0.01). Serum Amy, ALT and Cr levels were reduced, (Amy: 25.391.93 mkat, 37.66 ± 4.27 μkat, 96.00 ± 9.19 μkat; ALT: 94.5 ± 58.3 nkat/L, 283.8 ± 115.5 nkat/L, 469.7 ± 157.3 nkat/L; Cr: 81.4 ± 17.7 μmol/L, 89.1 ± 18.7 μmol/L, 110.7 ± 12.8 μmol/L, P < 0.05 or P < 0.01), and the pathological changes of pancreas were ameliorated by Oxy.
CONCLUSION: CINC and MCP-1/JE might play important roles in early ANP. The expression of CINC and MCP-1/JE gene in the pancreas can be inhibited by Oxy. Oxy therapy was effective in experimental ANP.
- Citation: Li ZS, Xu YC, Tu ZX, Shi XG. Chemokine gene expression in pancreatic tissue and effects of oxymatrine in early acute necrotizing pancreatitis. Shijie Huaren Xiaohua Zazhi 2005; 13(8): 979-983
- URL: https://www.wjgnet.com/1009-3079/full/v13/i8/979.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i8.979
重症急性胰腺炎(severe acute pancreatitis, SAP)发病后, 腺泡细胞所释放的炎症递质是此后细胞因子级链反应的触发剂, 导致白细胞激活, 并迁移、聚集至局部炎症组织, 释放大量破坏性炎性细胞因子, 吸收入血液循环后可能诱发全身炎症反应综合征(systemic inflammatory response syndrome, SIRS)、甚至多脏器功能衰竭(multiple organ failure, MOF), 成为SAP早期重要死因[1-5], 包括趋化因子在内的多种炎症递质参与了这一过程[6]. 我们运用大鼠急性坏死性胰腺炎(acute necrotizing pancreatitis, ANP)模型, 观察细胞因子诱导的中性粒细胞趋化因子(cytokine-induced neutrophil chemoattractant, CINC)和单核细胞趋化蛋白(monocyte chemotactic protein-1/JE, MCP-1/JE)在胰腺组织中的表达, 并探讨中药提取物氧化苦参碱(oxymatrine, Oxy)对他们的影响及在ANP中的治疗作用.
健康雄性SD大鼠, 上海第二军医大学实验动物中心提供, 体质量200-250 g; 牛磺胆酸钠购自美国Sigma公司; 苦参素注射液(含氧化苦参碱980 g/L)100 g/L, 宁夏制药厂生产; 宝生物工程(大连)有限公司的一步反应法RT-PCR试剂盒; CINC、MCP-1/JE及内参照β-actin引物根据GenBank中基因序列自行设计, 由上海生工生物工程公司合成.
实验动物56只随机分为假手术(sham operation, SO)组, 急性坏死性胰腺炎(acute necrotizing pancreatitis, ANP)3 h, 6 h及12 h组, 氧化苦参碱(oxymatrine, Oxy)治疗3 h, 6 h及12 h组, 每组各8只. Oxy治疗组以Oxy 50 mg/kg稀释后ip, 2次/d, 连续2 d, d3开始实验, 在诱导ANP后1 h以Oxy 50 mg/kg稀释后ip, SO组和ANP各组同法腹腔注入等量生理盐水. ANP模型制作如下: 大鼠术前禁食12 h和禁水6 h, 麻醉后进腹, 先用无损伤动脉夹夹闭肝门部胆胰管, 再用导管从十二指肠乳头对侧十二指肠肠壁穿刺入肠腔并插入胆胰管约1 cm, 微量泵以0.1 mL/min速度推注牛磺胆酸钠1 mL/kg, 注射结束后观察10 min拔管, 去除动脉夹, 缝合十二指肠穿刺口并关腹. SO组开腹后逆行胆胰管注射生理盐水1 mL/kg, 其余步骤与上述相同. 按时间点在处死大鼠前取血, 采用酶法(麦芽6糖)检测血清(Amy), 化学比色法检测(ALT), 酶法检测(Cr), 按Hitachi 7600型全自动生化分析仪(日本)标准规程操作, 用以评价大鼠胰腺及肝肾功能损伤程度.
1.2.1 胰腺组织病理学评价: 大鼠处死后取部分胰腺组织, 40 g/L中性缓冲甲醛固定, 石蜡包埋切片后HE染色制片, 每张切片随机选10个高倍镜视野(×400), 观察胰腺组织病理学变化及白细胞计数, 按下表胰腺组织损伤病理学评分标准评分(表1), 无表中各项病理改变计0分.
| 病理分级 | 评分 | 病理改变 |
| 水肿 | 1 | 小叶间区域性水肿, 叶间裂增宽 |
| 2 | 小叶间弥漫性水肿, 小叶内间隙增宽 | |
| 3 | 小叶间歇增大, 腺泡肿胀分离 | |
| 炎细胞浸润 | 1 | 白细胞<20 个/高倍镜视野 |
| 2 | 白细胞20-50个/高倍镜视野 | |
| 3 | 白细胞>30个/高倍镜视野或出现微脓肿 | |
| 出血 | 1 | 实质出血<20% |
| 2 | 实质出血20%-50% | |
| 3 | 实质出血>50% | |
| 坏死 | 1 | 坏死面积<20% |
| 2 | 坏死面积20%-50% | |
| 3 | 坏死面积 >50% |
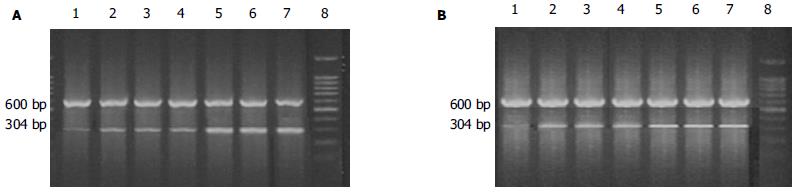
1.2.2 表1胰腺组织中CINC m RNA及MCP-1/JE mRNA的表达: 胰腺组织总RNA的抽提, 按Trizol液的说明书进行. RT-PCR反应体系均为50 μL, 50℃ RT反应60 min后PCR反应(表2). 取产物10 μL用15 g/L琼脂糖凝胶电泳, 紫外灯下以凝胶图像扫描仪测吸光度A值, 用β-actin校正半定量, 计算CINC mRNA和MCP-1/JE mRNA表达水平.
| 引物(产物大小) | 上游、下游 PCR | 反应条件 |
| CINC(304 bp) | 5'-GCTCGCTTCTCTGTGCAGC-3' 5'-CCATCGGTGCAATCTATCTTC-3' | 95℃变性5 min; 按94℃ 30 s, 62℃ 30 s, 72℃ 30 s, 35个循环; 72℃延伸5 min |
| MCP-1/JE(290 bp) | 5'-TTCACTGGCAAGATCCC-3' 5'-TGCTTGAGGTGGTTGTGGAA-3' | 95℃变性5 min; 按94℃ 30 s, 60℃ 30 s, 70℃ 30 s, 35个循环; 72℃延伸5 min |
| β-actin(600 bp) | 5'-AGGGTGTGATGGTGGGTATG-3' 5'-CATAGCTCTTCTCCAGGGAG-3' | 95℃变性5 min; 按94℃ 30 s, 62℃ 30 s, 72℃ 30 s, 35个循环; 72℃延伸5 min |
统计学处理 采用SPSS10.0统计软件进行分析, 组织学资料用评分积分值, 实验数据均用mean±SD表示, 两组间均数比较用单因素方差分析, 两变量间关系用直线相关分析.
与SO组相比, Oxy和ANP各组血清Amy随时间延长有显著升高趋势(P<0.01), Oxy各治疗组与ANP相应各组比较有明显降低(P<0.01). 比较SO组, ANP各组则ALT, Cr值显著升高(P<0.05或P<0.01), Oxy各组血清ALT, Cr值升高程度相对较轻, 与ANP各组比较有显著降低(P<0.05或P<0.01, 表3).
| 分组 | Amy(mkat)/L | ALT(nkat/L) | Cr(mmol/L) | 病理评分(分) |
| SO | 17.07±0.85 | 38.3±5.5 | 70.3±15.5 | 0.12±0.05 |
| ANP 3 h | 48.56±9.37b | 139.5±58.3b | 87.4±21.6a | 8.75±0.38b |
| ANP 6 h | 97.57±11.34b | 353.8±115.5b | 94.6±13.3b | 9.05±0.53b |
| ANP12 h | 159.13±18.07b | 589.7±157.3b | 131.7±20.5b | 10.15±0.64b |
| Oxy 3 h | 25.39±1.93bd | 94.5±58.3bd | 81.4±17.7bc | 5.95±0.17bd |
| Oxy 6 h | 37.66±4.27bd | 283.8±115.5bd | 89.1±18.7bc | 6.53±0.19bd |
| Oxy12 h | 96.00±9.19bd | 469.7±157.3bd | 110.7±12.8bd | 7.58±0.20bd |
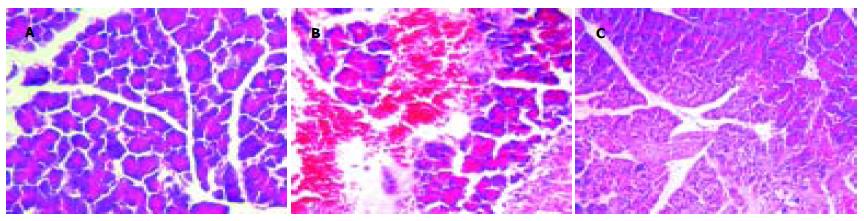
SO组胰腺外观正常; 诱发ANP 3 h后大鼠胰腺肿胀明显, 质地尚软; 6 h后质地变硬, 可见黄色坏死灶, 腹腔有少量血性腹水; 12 h后胰腺质硬, 见大片黄色坏死灶, 大网膜、肠系膜上见大量皂化斑, 腹腔有中至大量血性腹水; Oxy各组胰腺肿胀, 质地较硬, 坏死灶少见, 腹腔少至中量血性腹水. 显微镜下, SO组胰腺组织正常; 诱发ANP 3 h后胰腺间质水肿, 小叶间隙增大, 伴少量炎细胞浸润, 腺泡肿胀; 6 h后胰腺间质水肿, 中量炎细胞浸润, 有胰腺腺泡小叶结构破坏, 胰腺组织可见凝固性或脂肪坏死; 12 h后胰腺腺泡小叶结构破坏, 胰腺组织大片出血、凝固性坏死及脂肪坏死, 可见血管破裂出血, 坏死区见大量中性白细胞和单核细胞浸润; Oxy 3 h组胰腺间质水肿, 伴中等量炎细胞浸润, 胰腺腺泡有少量破坏, 或胰腺腺泡和导管基本正常, 6 h, 12 h后有胰腺腺泡小叶结构破坏, 胰腺组织坏死少见, 未见血管破裂出血, 坏死区有中量中性白细胞和单核细胞浸润. Oxy各组胰腺组织病理学改变较ANP各组有显著改善(P<0.01, 表3, 图1A-C).
SO组胰腺组织中CINC mRNA和MCP-1/JE mRNA表达量少, 相比较ANP各组CINC mRNA和MCP-1/JE mRNA表达量, 均随时间延长呈逐渐升高趋势(P<0.01), ANP 12 h时达高峰; Oxy各组CINC mRNA和MCP-1/JE mRNA表达量, 与SO组比较也随时间延长呈逐渐升高趋势(P<0.01), 比较ANP各组则有明显降低(P<0.01, 表4; 图2A-B).
胰腺组织中CINC mRNA和MCP-1/JE mRNA的表达与胰腺组织病理变化呈正相关, 相关系数分别为0.89和0.82(P<0.05).
急性胰腺炎(acute pancreatitis, AP)发病后, 尽管原发的病因可能已停止或去除, 但胰腺局部和全身反应仍可继续发展甚或恶化. 近年研究表明, 被激活的白细胞以及其释放肿瘤坏死因子-α, 白介素家族成员等炎症递质, 是造成胰腺局部损伤和诱发SIRS[7-8]甚至MOF的重要因素之一[9-11]. 这一过程有趋化因子等多种炎症递质的参与. 趋化因子分为CC, CXC, CX3C族和C亚族[12-14]. CINC与人类白介素-8(IL-8)超家族成员GRO-α同源, 是鼠CXC趋化因子的典型代表[15], CINC特异性的趋化中性粒细胞, 介导其跨越血管内皮, 激活中性粒细胞的呼吸爆发和脱颗粒, 从而诱导细胞膜表面表达黏附分子CD11b/CD18, 使中性粒细胞结合内皮细胞的能力增加3-10倍[16]. CINC在AP并发症的发病机制, 以及调节AP严重程度和导致AP相关的急性肺损伤等方面起重要作用. 趋化因子CC亚族中的MCP-1, 在败血症和其他一些炎症反应中起关键作用[17]. 研究表明, 在AP早期阶段, MCP-1即在腺泡细胞中产生, 同时在血清中水平显著升高, 比较CC亚族成员中的MIP-1α、MIP-1β等, MCP-1在AP局部并发症和远处脏器损害的发病机制中起关键作用[18]. MCP-1/JE是人类MCP-1在大鼠中的同源体, 是强有力的单核细胞趋化因子和激活剂[16]. 本研究中SO组大鼠胰腺组织中CINC mRNA和MCP-1/JE mRNA表达量很少, 随着ANP的诱发, RT-PCR检测提示, CINC mRNA和MCP-1/JE mRNA在ANP大鼠胰腺组织中的水平有显著增加, 随时间延长呈上调趋势, 并且与胰腺组织病理变化呈正相关. 提示CINC和MCP-1/JE在ANP大鼠胰腺组织中高表达, 促进了中性粒细胞和单核巨噬细胞的聚集, 加重胰腺组织局部的损害, CINC和MCP-1/JE是ANP早期重要的炎性细胞因子.
苦参型生物碱是一种具有苦参次碱-15-酮基本结构的化合物, 主要从苦参根及苦豆子中提取的有效成分, 《本草纲目》中记载: 苦参, 苦、寒、无毒、主治心腹结气, 症瑕积聚、黄疸、溺有余沥、逐水、补中明目, 养肝胆气, 并具有清热解毒祛风燥湿等功效. 氧化苦参碱是其中一种主要生物碱, 现代研究表明其具有抗菌[18]、保护肝细胞、抗肝纤维化[19-22]、抗炎、免疫调节等[23-25]药理作用, 能抑制TNF-α, IL-1和IL-6等炎性细胞因子的产生. 在抗病毒实验研究中, 运用剂量为100至300 mg/kg的Oxy被发现具有抑制乙型肝炎和丙型肝炎病毒的作用[19-22], 并且已经在临床中初步用于慢性乙型和慢性丙型肝炎的治疗[23-25]. 在调节免疫方面, 氧化苦参碱被认为低浓度能刺激免疫反应, 高浓度则抑制免疫反应, 而研究较多集中在其抑制免疫反应方面[26]. 其抗炎作用机制方面则被认为与抑制细胞因子的产生有关[27-28]. 目前, 氧化苦参碱应用在AP的治疗以及拮抗AP的全身炎症反应、调节免疫等方面研究报道很少. 本研究表明Oxy能明显抑制ANP大鼠胰腺组织中趋化因子CINC和MCP-1/JE的产生, 明显降低血清Amy, Cr, ALT水平, 减轻胰腺组织病理损伤指标, 可能与Oxy的抗炎作用、免疫抑制为主的调节作用、细胞膜稳定作用有关, 但将其在临床中运用的价值还有待于进一步研究.
编辑: 张海宁 电编: 潘伯荣
| 1. | Tao J, Wang C, Chen L, Yang Z, Xu Y, Xiong J, Zhou F. Diagnosis and management of severe acute pancreatitis complicated with abdominal compartment syndrome. J Huazhong Univ Sci Technolog Med Sci. 2003;23:399-402. [PubMed] |
| 2. | Pupelis G, Austrums E, Snippe K. Importance of a clinical protocol in the treatment of severe acute pancreatitis. Zentralbl Chir. 2002;127:975-981. [PubMed] |
| 3. | Wang H, Li WQ, Zhou W, Li N, Li JS. Clinical effects of continuous high volume hemofiltration on severe acute pancreatitis complicated with multiple organ dysfunction syndrome. World J Gastroenterol. 2003;9:2096-2099. [PubMed] |
| 4. | Zhu AJ, Shi JS, Sun XJ. Organ failure associated with severe acute pancreatitis. World J Gastroenterol. 2003;9:2570-2573. [PubMed] |
| 5. | Tao HQ, Zhang JX, Zou SC. Clinical characteristics and management of patients with early acute severe pancreatitis: experience from a medical center in China. World J Gastroenterol. 2004;10:919-921. [PubMed] |
| 6. | Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets Inflamm Allergy. 2002;1:343-351. [PubMed] |
| 7. | Naskalski JW, Kusnierz-Cabala B, Panek J, Kedra B. Poly-C specific ribonuclease activity correlates with increased concentrations of IL-6, IL-8 and sTNFR55/sTNFR75 in plasma of patients with acute pancreatitis. J Physiol Pharmacol. 2003;54:439-448. [PubMed] |
| 8. | Pooran N, Indaram A, Singh P, Bank S. Cytokines (IL-6, IL-8, TNF): early and reliable predictors of severe acute pancreatitis. J Clin Gastroenterol. 2003;37:263-266. [PubMed] |
| 9. | Bentrem DJ, Joehl RJ. Pancreas: healing response in critical illness. Crit Care Med. 2003;31:S582-S589. [PubMed] |
| 10. | Bhatia M, Proudfoot AE, Wells TN, Christmas S, Neoptolemos JP, Slavin J. Treatment with Met-RANTES reduces lung injury in caerulein-induced pancreatitis. Br J Surg. 2003;90:698-704. [PubMed] |
| 11. | Rau B, Baumgart K, Kruger CM, Schilling M, Beger HG. CC-chemokine activation in acute pancreatitis: enhanced release of monocyte chemoattractant protein-1 in patients with local and systemic complications. Intensive Care Med. 2003;29:622-629. [PubMed] |
| 12. | Gomez-Cambronero LG, Sabater L, Pereda J, Cassinello N, Camps B, Vina J, Sastre J. Role of cytokines and oxidative stress in the pathophysiology of acute pancreatitis: therapeutical implications. Curr Drug Targets Inflamm Allergy. 2002;1:393-403. |
| 13. | Pooran N, Indaram A, Singh P, Bank S. Cytokines (IL-6, IL-8, TNF): early and reliable predictors of severe acute pancreatitis. J Clin Gastroenterol. 2003;37:263-266. |
| 14. | Rau B, Baumgart K, Kruger CM, Schilling M, Beger HG. CC-chemokine activation in acute pancreatitis: enhanced release of monocyte chemoattractant protein-1 in patients with local and systemic complications. Intensive Care Med. 2003;29:622-629. |
| 15. | Brady M, Bhatia M, Christmas S, Boyd MT, Neoptolemos JP, Slavin J. Expression of the chemokines MCP-1/JE and cytokine-induced neutrophil chemoattractant in early acute pancreatitis. Pancreas. 2002;25:260-269. [PubMed] |
| 16. | Osman MO, Gesser B, Mortensen JT, Matsushima K, Jensen SL, Larsen CG. Profiles of pro-inflammatory cytokines in the serum of rabbits after experimentally induced acute pancreatitis. Cytokine. 2002;17:53-59. [PubMed] |
| 17. | Bhatia M, Brady M, Kang YK, Costello E, Newton DJ, Christmas SE, Neoptolemos JP, Slavin J. MCP-1 but not CINC synthesis is increased in rat pancreatic acini in response to cerulein hyperstimulation. Am J Physiol Gastrointest Liver Physiol. 2002;282:G77-85. [PubMed] |
| 18. | Rau B, Baumgart K, Kruger CM, Schilling M, Beger HG. CC-chemokine activation in acute pancreatitis: enhanced release of monocyte chemoattractant protein-1 in patients with local and systemic complications. Intensive Care Med. 2003;29:622-629. [PubMed] |
| 19. | Lu LG, Zeng MD, Mao YM, Fang JY, Song YL, Shen ZH, Cao AP. Inhibitory effect of oxymatrine on serum hepatitis B virus DNA in HBV transgenic mice. World J Gastroenterol. 2004;10:1176-1179. [PubMed] |
| 20. | Chen XS, Wang GJ, Cai X, Yu HY, Hu YP. Inhibition of hepatitis B virus by oxymatrine in vivo. World J Gastroenterol. 2001;7:49-52. [PubMed] |
| 21. | Liu J, Manheimer E, Tsutani K, Gluud C. Medicinal herbs for hepatitis C virus infection: a Cochrane hepatobiliary systematic review of randomized trials. Am J Gastroenterol. 2003;98:538-544. [PubMed] |
| 22. | Chen Y, Li J, Zeng M, Lu L, Qu D, Mao Y, Fan Z, Hua J. The inhibitory effect of oxymatrine on hepatitis C virus in vitro. Zhonghua Ganzangbing Zazhi. 2001;9:12-14. [PubMed] |
| 23. | Lu LG, Zeng MD, Mao YM, Li JQ, Wan MB, Li CZ, Chen CW, Fu QC, Wang JY, She WM. Oxymatrine therapy for chronic hepatitis B: a randomized double-blind and placebo-controlled multi-center trial. World J Gastroenterol. 2003;9:2480-2483. [PubMed] |
| 24. | Chen YX, Mao BY, Jiang JH. Relationship between serum load of HBV-DNA and therapeutic effect of oxymatrine in patients with chronic hepatitis B. Zhongguo Zhongxiyi Jiehe Zazhi. 2002;22:335-336. [PubMed] |
| 25. | Yu YY, Wang QH, Zhu LM, Zhang QB, Xu DZ, Guo YB, Wang CQ, Guo SH, Zhou XQ, Zhang LX. A clinical research on oxymatrine for the treatment of chronic hepatitis B. Zhonghua Ganzangbing Zazhi. 2002;10:280-281. [PubMed] |
| 26. | Dong Y, Xi H, Yu Y, Wang Q, Jiang K, Li L. Effects of oxymatrine on the serum levels of T helper cell 1 and 2 cytokines and the expression of the S gene in hepatitis B virus S gene transgenic mice: a study on the anti-hepatitis B virus mechanism of oxymatrine. J Gastroenterol Hepatol. 2002;17:1299-1306. [PubMed] |
| 27. | Xiang X, Wang G, Cai X, Li Y. Effect of oxymatrine on murine fulminant hepatitis and hepatocyte apoptosis. Chin Med J (Engl). 2002;115:593-596. [PubMed] |
| 28. | Yang W, Zeng M, Fan Z, Mao Y, Song Y, Jia Y, Lu L, Chen CW, Peng YS, Zhu HY. Prophylactic and therapeutic effect of oxymatrine on D-galactosamine-induced rat liver fibrosis. Zhonghua Ganzangbing Zazhi. 2002;10:193-196. [PubMed] |