INTRODUCTION
Malignant fibrous histiocytoma (MFH) is the most common type of soft tissue sarcoma of middle and late adulthood[1–4], but it originates only infrequently in the pancreas[5–8]. Since the first MFH of the pancreas was described in 1976 by Margules et al[9], only 20 cases have been documented in the world literature, including the present case[10–13]. Some of these cases have exhibited the characteristic radiological findings[10–12], and no case with recurrence has been reported. Herein, we present a patient with primary MFH of the pancreas, which recurred 6 mo after surgery, and its computed tomography (CT) and magnetic resonance imaging (MRI) appearance. To the best of our knowledge, this is the first report on recurrence of primary MFH of the pancreas and the first with MRI findings.
CASE REPORT
A 67-year-old man was referred to the hospital because of abnormal ultrasonographic findings in the pancreas, found by chance at medical checkup. He was asymptomatic apart from a 3-kg decrease in body weight over the past 6 mo. The patient had no history of alcohol consumption or smoking. Physical examination on admission was unremarkable except for a 9 cm × 10 cm hard mass located in the middle abdomen. No lymph nodes were detected at the body surface. Hepatitis B and C serology was negative, and blood cell counts, liver function tests and serum amylase were normal. Serum levels of carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) were not elevated.
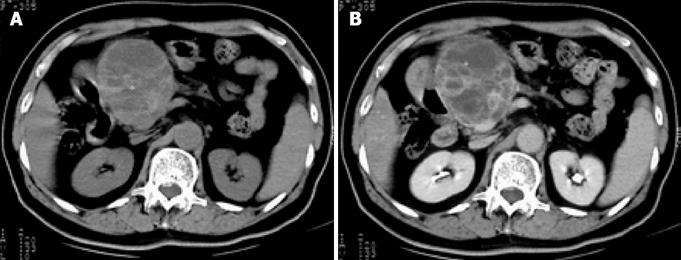
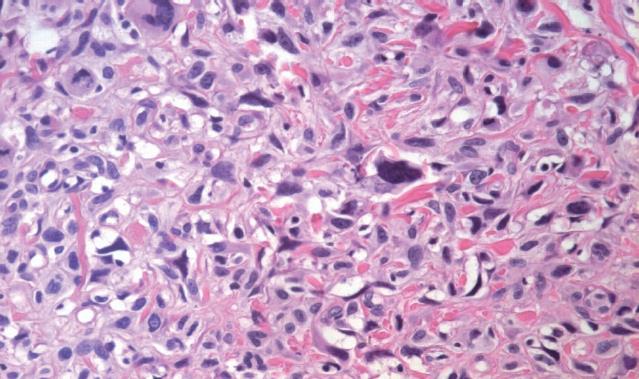
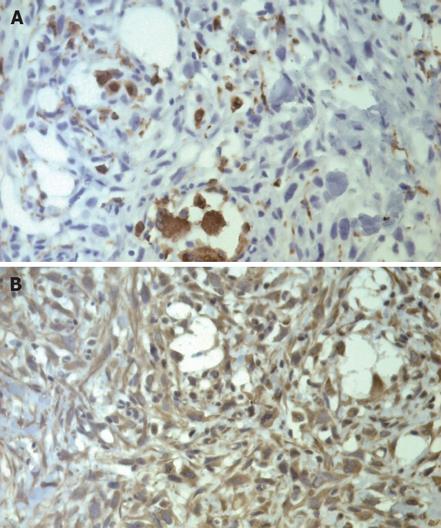
Abdominal ultrasound showed a huge soft-tissue mass with cystic and solid components in the head of the pancreas. Abdominal CT revealed a large (8.2 cm × 9.5 cm), non-homogeneous, dense mass in the head of the pancreas, with obvious atrophy in the body and tail of the pancreas on pre-contrast CT (Figure 1A). The lesion showed a well-defined, multilocular cystic mass with a cystic wall, fibrous septa and enhancement of solid components on post-contrast CT (Figure 1B). None of the imaging examinations showed abnormal findings in the liver, spleen or retroperitoneum. There was no evidence of metastatic disease, and the patient underwent pancreaticoduodenectomy. Intraoperative findings demonstrated a large mass in the head of the pancreas and no adhesion to the liver or retroperitoneum. Macroscopically, the tumor specimen measured 10 cm × 11 cm × 8 cm in size, and was partially encapsulated by the normal pancreas. The cut surface of the tumor was gray-white in color, with a large area of hemorrhage and necrosis. Microscopically, the tumor was composed of spindle cells arranged in a storiform pattern with varying numbers of polymorphic neoplastic and multinucleated cells (Figure 2). To clarify the diagnosis of the tumor further, immunoperoxidase staining was performed on the tumor specimen. The tumor cells were negative for desmin, CEA and S-100; positive for CD68 (Figure 3A), vimentin (Figure 3B), lysozyme and α-1-ACT; and no evidence of epithelial differentiation was found. In summary, the diagnosis of storiform-pleomorphic MFH of the pancreas was made.
Figure 1 A: Pre-contrast CT shows a large, non-homogeneous, density mass in the head of the pancreas, with obvious atrophy in the body and tail of the pancreas; B: Lesion illustrates a well-defined, multilocular cystic mass with a cystic wall, fibrous septa and enhancement of solid components on post-contrast CT.
Figure 2 Pathology of the pancreatic mass shows spindle cells arranged in a storiform pattern, with varying numbers of polymorphic neoplastic and multinucleated cells (HE, × 200).
Figure 3 Immunohistochemical staining shows that the tumor cells are positive for CD68 (A) and vimentin (B) (× 250).
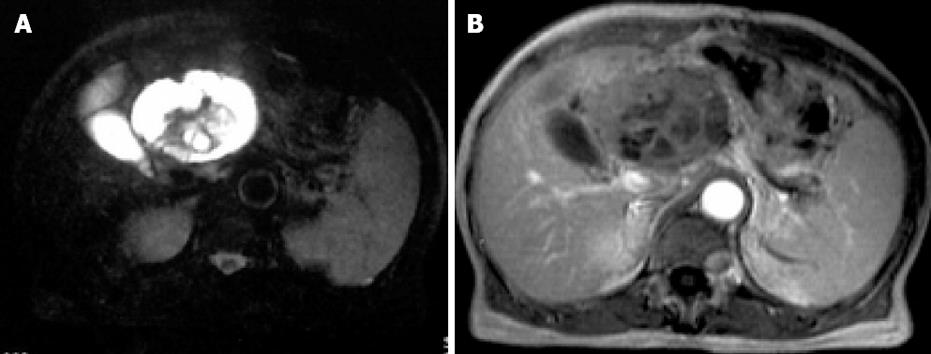
The patient enjoyed a smooth recovery and was discharged on postoperative d 10. The patient was followed at 2-mo intervals by abdominal ultrasound. There was no evidence of recurrence or metastasis until 6 mo postoperatively. MRI demonstrated that a new mass had developed at the location of the MFH. T1-weighted imaging showed a large (7 cm × 8.5 cm), non-heterogeneous, hypointense lesion of the liver. Fat-saturated T2-weighted imaging showed non-heterogeneous hyperintensity in the liver, with a hypointense area in the central part of the tumor (Figure 4A). Contrast-enhanced T1-weighted imaging demonstrated a large multilocular cystic mass with a cystic wall, fibrous septa and enhancement of solid components (Figure 4B). A diagnosis of recurrence was made. The patient died from the recurrence at 11 mo after operation.
Figure 4 A: MR fat-saturated T2-weighted image shows a large, nonheterogeneous, hyperintense lesion in the liver, with a hypointense area in the central part of the tumor; B: Contrast-enhanced T1-weighted image shows a large multilocular cystic mass with a cystic wall, fibrous septa and enhancement of solid components.
DISCUSSION
MFH accounts for > 30% of all soft tissue sarcomas[14], and occurs most frequently in the extremities, trunk and retroperitoneum[15–18]. Men are affected twice as frequently as women[1519]. Primary MFH of the pancreas is exceedingly rare[5–8]. Akatsu et al have reviewed 16 cases and reported that the tumor has a male predominance with a median age at onset of 55 years. It is usually a large mass (mean tumor diameter is 13 cm, with a range of 4-35 cm)[11]. Of the documented cases, only three survived for > 48 mo[10–13], which suggests a poor prognosis for long-term survival. Only one case had lymph node metastases[12]. Our case was the only one with recurrence.
MFH consists of histiocyte-like and fibroblast-like cells arranged in a storiform pattern, with other pleomorphic cells and multinucleated giant cells[20–23]. There are five subtypes of MFH according to histology[1024]: storiform-pleomorphic, myxoid, giant cell, inflammatory, and angiomatoid. The storiform-pleomorphic pattern is the most common variant[25]. Three histological subtypes have been observed among the 20 cases previously reported in the literature: 13 (65%) storiform-pleomorphic, four (20%) giant cell, and three (15%) myxoid[2–5]. Our case belonged to the storiform-pleomorphic type.
Only a few radiological findings of primary MFH of the pancreas have been described[10–12]. The CT findings of the tumor include the following: a large, heterogeneous, low-attenuation density or multinodular mass, with intratumoral calcification on pre-contrast CT; non-homogeneously enhancing pancreatic mass with a large amount of necrosis[1112]; and single enhancing peripheral pseudo-capsular mass on post-contrast CT[10]. To the best of our knowledge, we describe the first case of primary MFH of the pancreas, with CT showing a large multilocular cystic mass with a cystic wall, fibrous septa and enhancement of solid components. It is not clear whether or not the pathological features of pancreatic MFH correlate with the CT findings. Liu et al have described a predominant cystic MFH of the pancreas of the myxoid variety[10], but other reports and our case suggest that not all cystic degeneration of the tumor belongs to the myxoid pattern[1112]. Ko et al have considered that abdominal MFH, especially the storiform-pleomorphic subtype, exhibits metaplastic calcifications on CT, and they showed a heterogeneously enhanced mass with a large necrotic area in the pancreatic body and tail, and eccentrically located ring calcification[26]. Akatsu et al did not, however, support this sign and concluded that calcification is not always helpful in differentiating pancreatic MFH from other pancreatic tumors[11]. Therefore, we think that more cases need to be studied to prove the relationship between the CT findings and the subtypes of primary MFH.
Mahajan and colleagues have retrospectively reviewed the MRI changes in 39 patients with MFH, however, none of these originated from the pancreas[27]. This study concluded that MRI sensitivity and specificity for detecting a neoplasm were 96% and 83%, respectively, but the signal changes were non-specific for MFH. When compared to CT in 14 patients, MRI better defined the extent of the MFH, its relationship to surrounding tissues and vessels, and best differentiated residual or recurrent disease from postoperative changes when examined at least 3 mo after surgery. There was no significant difference in signal intensity of 12 preoperative and 13 recurrent neoplasms[27].
Tateishi et al have described the manner in which that primary MFH has a high signal intensity on T2-weighted MR images and non-homogeneous isosignal intensity compared with the surrounding muscle[28]. Miller et al have reported that a correlation between MRI appearance and histopathology may not be established, but concluded that MRI exhibits general features suggestive of malignant soft tissue neoplasm, namely, internal low signal septation, and heterogeneous high signal intensity on T2-weighted images[29]. Kitajima et al[30] have considered that MRI is helpful in the differential diagnosis between MFH of the renal capsule and other renal tumors, and that a hypointense area identified on T2-weighted images is an important characteristic of renal MFH. In our case, MRI showed a large multilocular cystic mass, which was similar to that on preoperative CT, and it could reveal the exact location and extent of the tumor recurrence. An obviously hypointense area on T2-weighted images that reflected the fibrous component was also seen in our pancreatic MFH.
In conclusion, primary MFH of the pancreas is rare and a distinct clinical entity. It may recur and the prognosis is poor. Although there are no unique findings of pancreatic MFH, a large, massive liquescent necrotic mass or multilocular cystic lesion, with calcification on CT or a hypointense area within the mass on T2-weighted imaging in an older male patient, should lead to consideration of a differential diagnosis of pancreatic MFH.